Dolutegravir twice-daily dosing in children with HIV-associated tuberculosis: a pharmacokinetic and safety study within the open-label, multicentre, randomised, non-inferiority ODYSSEY trial
- PMID: 35868341
- PMCID: PMC9630157
- DOI: 10.1016/S2352-3018(22)00160-6
Dolutegravir twice-daily dosing in children with HIV-associated tuberculosis: a pharmacokinetic and safety study within the open-label, multicentre, randomised, non-inferiority ODYSSEY trial
Abstract
Background: Children with HIV-associated tuberculosis (TB) have few antiretroviral therapy (ART) options. We aimed to evaluate the safety and pharmacokinetics of dolutegravir twice-daily dosing in children receiving rifampicin for HIV-associated TB.
Methods: We nested a two-period, fixed-order pharmacokinetic substudy within the open-label, multicentre, randomised, controlled, non-inferiority ODYSSEY trial at research centres in South Africa, Uganda, and Zimbabwe. Children (aged 4 weeks to <18 years) with HIV-associated TB who were receiving rifampicin and twice-daily dolutegravir were eligible for inclusion. We did a 12-h pharmacokinetic profile on rifampicin and twice-daily dolutegravir and a 24-h profile on once-daily dolutegravir. Geometric mean ratios for trough plasma concentration (Ctrough), area under the plasma concentration time curve from 0 h to 24 h after dosing (AUC0-24 h), and maximum plasma concentration (Cmax) were used to compare dolutegravir concentrations between substudy days. We assessed rifampicin Cmax on the first substudy day. All children within ODYSSEY with HIV-associated TB who received rifampicin and twice-daily dolutegravir were included in the safety analysis. We described adverse events reported from starting twice-daily dolutegravir to 30 days after returning to once-daily dolutegravir. This trial is registered with ClinicalTrials.gov (NCT02259127), EudraCT (2014-002632-14), and the ISRCTN registry (ISRCTN91737921).
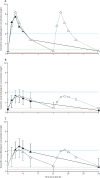
Findings: Between Sept 20, 2016, and June 28, 2021, 37 children with HIV-associated TB (median age 11·9 years [range 0·4-17·6], 19 [51%] were female and 18 [49%] were male, 36 [97%] in Africa and one [3%] in Thailand) received rifampicin with twice-daily dolutegravir and were included in the safety analysis. 20 (54%) of 37 children enrolled in the pharmacokinetic substudy, 14 of whom contributed at least one evaluable pharmacokinetic curve for dolutegravir, including 12 who had within-participant comparisons. Geometric mean ratios for rifampicin and twice-daily dolutegravir versus once-daily dolutegravir were 1·51 (90% CI 1·08-2·11) for Ctrough, 1·23 (0·99-1·53) for AUC0-24 h, and 0·94 (0·76-1·16) for Cmax. Individual dolutegravir Ctrough concentrations were higher than the 90% effective concentration (ie, 0·32 mg/L) in all children receiving rifampicin and twice-daily dolutegravir. Of 18 children with evaluable rifampicin concentrations, 15 (83%) had a Cmax of less than the optimal target concentration of 8 mg/L. Rifampicin geometric mean Cmax was 5·1 mg/L (coefficient of variation 71%). During a median follow-up of 31 weeks (IQR 30-40), 15 grade 3 or higher adverse events occurred among 11 (30%) of 37 children, ten serious adverse events occurred among eight (22%) children, including two deaths (one tuberculosis-related death, one death due to traumatic injury); no adverse events, including deaths, were considered related to dolutegravir.
Interpretation: Twice-daily dolutegravir was shown to be safe and sufficient to overcome the rifampicin enzyme-inducing effect in children, and could provide a practical ART option for children with HIV-associated TB.
Funding: Penta Foundation, ViiV Healthcare, UK Medical Research Council.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AT, DF, DMG, and MKC received support from core funding to the Medical Research Council Clinical Trials Unit (grant numbers MCUU_00004/03 and MCUU_00004/07). AC received grants for pregnancy research from Gilead Sciences, ViiV Healthcare, and Merck Group. AT received funding for serving on ViiV Healthcare advisory board. AV served on a Data Safety Monitoring Board for Janssen Pharmaceuticals. DMB received grants from ViiV Healthcare, Gilead Sciences, and Merck Group for the PANNA network; served on the advisory board for Merck Group; participated in the work of the Data Safety Monitoring Board for Janssen Pharmaceuticals; and received funding from ViiV Healthcare and Pfizer for lectures. RAF received Senior fellowship in Clinical Science from Wellcome Trust. AT, AC, DMB, DMG, MA, and PA are members of the WHO-led Paediatric Antiretroviral Working Group (PAWG) and Paediatric Drug Optimisation Group (PADO). AT and PA are members of the WHO-led Child and Adolescent TB Technical Working Group. MA and AC served as PAWG Co-Chairs. MA served as a Vice Chair of the Treatment Scientific Committee of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT). All other authors declare no competing interests.
Figures
Comment in
-
Dolutegravir for children with HIV-associated tuberculosis.Lancet HIV. 2022 Sep;9(9):e599-e600. doi: 10.1016/S2352-3018(22)00172-2. Epub 2022 Jul 19. Lancet HIV. 2022. PMID: 35868340 No abstract available.
References
-
- WHO Global tuberculosis report 2021. October, 2021. https://www.who.int/publications/i/item/9789240037021
-
- Semvua HH, Kibiki GS, Kisanga ER, Boeree MJ, Burger DM, Aarnoutse R. Pharmacological interactions between rifampicin and antiretroviral drugs: challenges and research priorities for resource-limited settings. Ther Drug Monit. 2015;37:22–32. - PubMed
-
- WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. July, 2021. https://www.who.int/publications/i/item/9789240031593 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

