COVID-19 booster dose induces robust antibody response in pregnant, lactating, and nonpregnant women
- PMID: 35868417
- PMCID: PMC9295313
- DOI: 10.1016/j.ajog.2022.07.014
COVID-19 booster dose induces robust antibody response in pregnant, lactating, and nonpregnant women
Abstract
Background: Although emerging data during the SARS-CoV-2 pandemic have demonstrated robust messenger RNA vaccine-induced immunogenicity across populations, including pregnant and lactating individuals, the rapid waning of vaccine-induced immunity and the emergence of variants of concern motivated the use of messenger RNA vaccine booster doses. Whether all populations, including pregnant and lactating individuals, will mount a comparable response to a booster dose is not known.
Objective: This study aimed to profile the humoral immune response to a COVID-19 messenger RNA booster dose in a cohort of pregnant, lactating, and nonpregnant age-matched women.
Study design: This study characterized the antibody response against ancestral Spike and Omicron in a cohort of 31 pregnant, 12 lactating, and 20 nonpregnant age-matched controls who received a BNT162b2 or messenger RNA-1273 booster dose after primary COVID-19 vaccination. In addition, this study examined the vaccine-induced antibody profiles of 15 maternal-to-cord dyads at delivery.
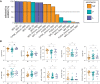
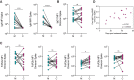
Results: Receiving a booster dose during pregnancy resulted in increased immunoglobulin G1 levels against Omicron Spike (postprimary vaccination vs postbooster dose; P=.03). Pregnant and lactating individuals exhibited equivalent Spike-specific total immunoglobulin G1, immunoglobulin M, and immunoglobulin A levels and neutralizing titers against Omicron compared with nonpregnant women. Subtle differences in Fc receptor binding and antibody subclass profiles were observed in the immune response to a booster dose in pregnant vs nonpregnant individuals. The analysis of maternal and cord antibody profiles at delivery demonstrated equivalent total Spike-specific immunoglobulin G1 in maternal and cord blood, yet higher Spike-specific FcγR3a-binding antibodies in the cord relative to maternal blood (P=.002), consistent with the preferential transfer of highly functional immunoglobulin. Spike-specific immunoglobulin G1 levels in the cord were positively correlated with the time elapsed since receiving the booster dose (Spearman R, .574; P=.035).
Conclusion: Study data suggested that receiving a booster dose during pregnancy induces a robust Spike-specific humoral immune response, including against Omicron. If boosting occurs in the third trimester of pregnancy, higher Spike-specific cord immunoglobulin G1 levels are achieved with greater time elapsed between receiving the booster and delivery. Receiving a booster dose has the potential to augment maternal and neonatal immunity.
Keywords: COVID-19; SARS-CoV-2; antibodies; booster; humoral immune response; immune response; immunity; messenger RNA vaccine; transplacental antibody transfer; vaccination.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Centers for Disease Control and Prevention . 2022. COVID-19 vaccines while pregnant or breastfeeding.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregn... Available at:
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI142790/AI/NIAID NIH HHS/United States
- U19 AI167899/AI/NIAID NIH HHS/United States
- R01 AI042790/AI/NIAID NIH HHS/United States
- R01 AI146785/AI/NIAID NIH HHS/United States
- DP2 DA040254/DA/NIDA NIH HHS/United States
- K12 HD103096/HD/NICHD NIH HHS/United States
- K08 HL146963/HL/NHLBI NIH HHS/United States
- 75N93019C00052/AI/NIAID NIH HHS/United States
- R01 HD100022/HD/NICHD NIH HHS/United States
- U19 AI135995/AI/NIAID NIH HHS/United States
- U01 CA260476/CA/NCI NIH HHS/United States
- R37 AI080289/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

