Placental transcriptomic signatures of spontaneous preterm birth
- PMID: 35868418
- PMCID: PMC9790028
- DOI: 10.1016/j.ajog.2022.07.015
Placental transcriptomic signatures of spontaneous preterm birth
Abstract
Background: Spontaneous preterm birth accounts for most preterm births and leads to significant morbidity in the newborn and childhood period. This subtype of preterm birth represents an increasing proportion of all preterm births when compared with medically indicated preterm birth, yet it is understudied in omics analyses. The placenta is a key regulator of fetal and newborn health, and the placental transcriptome can provide insight into pathologic changes that lead to spontaneous preterm birth.
Objective: This analysis aimed to identify genes for which placental expression was associated with spontaneous preterm birth (including early preterm and late preterm birth).
Study design: The ECHO PATHWAYS consortium extracted RNA from placental samples collected from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood and the Global Alliance to Prevent Prematurity and Stillbirth studies. Placental transcriptomic data were obtained by RNA sequencing. Linear models were fit to estimate differences in placental gene expression between term birth and spontaneous preterm birth (including gestational age subgroups defined by the American College of Obstetricians and Gynecologists). Models were adjusted for numerous confounding variables, including labor status, cohort, and RNA sequencing batch. This analysis excluded patients with induced labor, chorioamnionitis, multifetal gestations, or medical indications for preterm birth. Our combined cohort contained gene expression data for 14,023 genes in 48 preterm and 540 term samples. Genes and pathways were considered statistically significantly different at false discovery rate-adjusted P value of <.05.
Results: In total, we identified 1728 genes for which placental expression was associated with spontaneous preterm birth with more differences in expression in early preterm samples than late preterm samples when compared with full-term samples. Of those, 9 genes were significantly decreased in both early and late spontaneous preterm birth, and the strongest associations involved placental expression of IL1B, ALPL, and CRLF1. In early and late preterm samples, we observed decreased expression of genes involved in immune signaling, signal transduction, and endocrine function.
Conclusion: This study provides a comprehensive assessment of the differences in the placental transcriptome associated with spontaneous preterm birth with robust adjustment for confounding. Results of this study are in alignment with the known etiology of spontaneous preterm birth, because we identified multiple genes and pathways for which the placental and chorioamniotic membrane expression was previously associated with prematurity, including IL1B. We identified decreased expression in key signaling pathways that are essential for placental growth and function, which may be related to the etiology of spontaneous preterm birth. We identified increased expression of genes within metabolic pathways associated exclusively with early preterm birth. These signaling and metabolic pathways may provide clinically targetable pathways and biomarkers. The findings presented here can be used to understand underlying pathologic changes in premature placentas, which can inform and improve clinical obstetrics practice.
Keywords: ALPL; GABRP; IL1B; chemokine signaling; placenta; placental metabolism; signal transduction; spontaneous preterm birth; transcriptomics.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


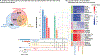

References
-
- Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotech. 2016;34(5):525–527. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

