Cytochrome P450 oxidase 2J inhibition suppresses choroidal neovascularization in mice
- PMID: 35868524
- PMCID: PMC9535696
- DOI: 10.1016/j.metabol.2022.155266
Cytochrome P450 oxidase 2J inhibition suppresses choroidal neovascularization in mice
Abstract
Introduction: Choroidal neovascularization (CNV) in age-related macular degeneration (AMD) leads to blindness. It has been widely reported that increased intake of ω-3 long-chain polyunsaturated fatty acids (LCPUFA) diets reduce CNV. Of the three major pathways metabolizing ω-3 (and ω-6 LCPUFA), the cyclooxygenase and lipoxygenase pathways generally produce pro-angiogenic metabolites from ω-6 LCPUFA and anti-angiogenic ones from ω-3 LCPUFA. Howevehr, cytochrome P450 oxidase (CPY) 2C produces pro-angiogenic metabolites from both ω-6 and ω-3 LCPUFA. The effects of CYP2J2 products on ocular neovascularization are still unknown. Understanding how each metabolic pathway affects the protective effect of ω-3 LCPUFA on retinal neovascularization may lead to therapeutic interventions.
Objectives: To investigate the effects of LCPUFA metabolites through CYP2J2 pathway and CYP2J2 regulation on CNV both in vivo and ex vivo.
Methods: The impact of CYP2J2 overexpression and inhibition on neovascularization in the laser-induced CNV mouse model was assessed. The plasma levels of CYP2J2 metabolites were measured by liquid chromatography and tandem mass spectroscopy. The choroidal explant sprouting assay was used to investigate the effects of CYP2J2 inhibition and specific LCPUFA CYP2J2 metabolites on angiogenesis ex vivo.
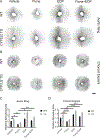
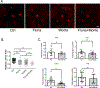
Results: CNV was exacerbated in Tie2-Cre CYP2J2-overexpressing mice and was associated with increased levels of plasma docosahexaenoic acids. Inhibiting CYP2J2 activity with flunarizine decreased CNV in both ω-6 and ω-3 LCPUFA-fed wild-type mice. In Tie2-Cre CYP2J2-overexpressing mice, flunarizine suppressed CNV by 33 % and 36 % in ω-6, ω-3 LCPUFA diets, respectively, and reduced plasma levels of CYP2J2 metabolites. The pro-angiogenic role of CYP2J2 was corroborated in the choroidal explant sprouting assay. Flunarizine attenuated ex vivo choroidal sprouting, and 19,20-EDP, a ω-3 LCPUFA CYP2J2 metabolite, increased sprouting. The combined inhibition of CYP2J2 with flunarizine and CYP2C8 with montelukast further enhanced CNV suppression via tumor necrosis factor-α suppression.
Conclusions: CYP2J2 inhibition augmented the inhibitory effect of ω-3 LCPUFA on CNV. Flunarizine suppressed pathological choroidal angiogenesis, and co-treatment with montelukast inhibiting CYP2C8 further enhanced the effect. CYP2 inhibition might be a viable approach to suppress CNV in AMD.
Keywords: Age-related macular degeneration; Choroidal neovascularization; Cytochrome P450 oxidase 2J; Lipid metabolism; Long-chain polyunsaturated fatty acid; Tumor necrosis factor-α.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest
All authors have no conflicts of interest.
Figures



References
-
- Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet 2018;392:1147–59. - PubMed
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16. - PubMed
-
- Jhingan M, Singh SR, Samanta A, Arora S, Tucci D, Amarasekera S, et al. Drusen ooze: predictor for progression of dry age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2021;259:2687–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

