Variation in TAF1 expression in female carrier induced pluripotent stem cells and human brain ontogeny has implications for adult neostriatum vulnerability in X-linked Dystonia Parkinsonism
- PMID: 35868859
- PMCID: PMC9428949
- DOI: 10.1523/ENEURO.0129-22.2022
Variation in TAF1 expression in female carrier induced pluripotent stem cells and human brain ontogeny has implications for adult neostriatum vulnerability in X-linked Dystonia Parkinsonism
Abstract
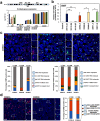
X-linked Dystonia-Parkinsonism (XDP) is an inherited, X-linked, adult-onset movement disorder characterized by degeneration in the neostriatum. No therapeutics alter disease progression. The mechanisms underlying regional differences in degeneration and adult onset are unknown. Developing therapeutics requires a deeper understanding of how XDP-relevant features vary in health and disease. XDP is possibly due, in part, to a partial loss of TAF1 function. A disease-specific SINE-VNTR-Alu (SVA) retrotransposon insertion occurs within intron 32 of TAF1, a subunit of TFIID involved in transcription initiation. While all XDP males are usually clinically affected, females are heterozygous carriers generally not manifesting the full syndrome. As a resource for disease modeling, we characterized eight iPSC lines from three XDP female carrier individuals for X chromosome inactivation status and identified clonal lines that express either the wild-type X or XDP haplotype. Furthermore, we characterized XDP-relevant transcript expression in neurotypical humans, and found that SVA-F expression decreases after 30 years of age in the brain and that TAF1 is decreased in most female samples. Uniquely in the caudate nucleus, TAF1 expression is not sexually dimorphic and decreased after adolescence. These findings indicate that regional-, age- and sex-specific mechanisms regulate TAF1, highlighting the importance of disease-relevant models and postmortem tissue. We propose that the decreased TAF1 expression in the adult caudate may synergize with the XDP-specific partial loss of TAF1 function in patients, thereby passing a minimum threshold of TAF1 function, and triggering degeneration in the neostriatum.Significance StatementXDP is an inherited, X-linked, adult-onset movement disorder characterized by degeneration in the neostriatum. No therapeutics alter disease progression. Developing therapeutics requires a deeper understanding of how XDP-relevant features vary in health and disease. XDP is possibly due to a partial loss of TAF1 function. While all XDP males are usually affected, females are heterozygous carriers generally not manifesting the full syndrome. As a resource for disease modeling, we characterized eight stem cell lines from XDP female carrier individuals. Furthermore, we found that, uniquely in the caudate nucleus, TAF1 expression decreases after adolescence in healthy humans. We hypothesize that the decrease of TAF1 after adolescence in human caudate, in general, may underlie the vulnerability of the adult neostriatum in XDP.
Keywords: SVA retrotransposon; X-linked Dystonia Parkinsonism; induced pluripotent stem cells; movement disorder; neurodegeneration; repeat expansion.
Copyright © 2022 D’Ignazio et al.
Conflict of interest statement
Authors report no conflict of interest
Figures




References
-
- Benjamin KJ, Feltrin AS, Barbosa AR, Jaffe AE, Stolz J, Collado-Torres L, Huuki-Myers LA, Burke E, Shin JH, Ulrich WS, Deep-Soboslay A, Tao R, Hyde TM, Kleinman JE, Erwin JA, Weinberger DR, Paquola AC (2020) Genetic and environmental regulation of caudate nucleus transcriptome: insight into schizophrenia risk and the dopamine system. medRxiv.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
