Immunogenicity of BNT162b2 COVID-19 vaccine in New Zealand adults
- PMID: 35868948
- PMCID: PMC9273612
- DOI: 10.1016/j.vaccine.2022.07.009
Immunogenicity of BNT162b2 COVID-19 vaccine in New Zealand adults
Abstract
Background: There is very little known about SARS-CoV-2 vaccine immune responses in New Zealand populations at greatest risk for serious COVID-19 disease.
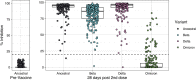
Methods: This prospective cohort study assessed immunogenicity in BNT162b2 mRNA vaccine recipients in New Zealand without previous COVID-19, with enrichment for Māori, Pacific peoples, older adults ≥ 65 years of age, and those with co-morbidities. Serum samples were analysed at baseline and 28 days after second dose for presence of quantitative anti-S IgG by chemiluminescent microparticle immunoassay and for neutralizing capacity against Wuhan, Beta, Delta, and Omicron BA.1 strains using a surrogate viral neutralisation assay.
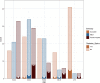
Results: 285 adults with median age of 52 years were included. 55% were female, 30% were Māori, 28% were Pacific peoples, and 26% were ≥ 65 years of age. Obesity, cardiac and pulmonary disease and diabetes were more common than in the general population. All participants received 2 doses of BNT162b2 vaccine. At 28 days after second vaccination, 99.6% seroconverted to the vaccine, and anti-S IgG and neutralising antibody levels were high across gender and ethnic groups. IgG and neutralising responses declined with age. Lower responses were associated with age ≥ 75 and diabetes, but not BMI. The ability to neutralise the Omicron BA.1 variant in vitro was severely diminished but maintained against other variants of concern.
Conclusions: Vaccine antibody responses to BNT162b2 were generally robust and consistent with international data in this COVID-19 naïve cohort with representation of key populations at risk for COVID-19 morbidity. Subsequent data on response to boosters, durability of responses and cellular immune responses should be assessed with attention to elderly adults and diabetics.
Keywords: COVID-19; Immunogenicity; Māori; Pacific Islander; Vaccine.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: James Ussher reports financial support was provided by New Zealand Ministry of Business Innovation and Employment, Michael Williams reports financial support was provided by Malaghan Institute of Medical Research, Simon Carson reports financial support was provided by Malaghan Institute of Medical Research, Graham Le Gros reports financial support was provided by New Zealand Ministry of Business Innovation and Employment, Julia Mathieson reports financial support was provided by Malaghan Institute of Medical Research, Frances H. Priddy reports financial support was provided by New Zealand Ministry of Business Innovation and Employment. Frances Priddy reports financial support was provided by New Zealand Ministry of Health. Frances Priddy reports a relationship with Janssen Pharmaceuticals Inc that includes: consulting or advisory.
Figures


References
-
- Steyn N., Binny R.N., Hannah K., Hendy S.C., James A., Lustig A., et al. Māori and pacific people in New Zealand have a higher risk of hospitalisation for COVID-19 | open access. N Z Med J. 2021;134:28–43. - PubMed
-
- COVID-19: Case demographics | Ministry of Health NZ n.d. https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-data-and-....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

