Nanomedicine Strategies for Heating "Cold" Ovarian Cancer (OC): Next Evolution in Immunotherapy of OC
- PMID: 35869032
- PMCID: PMC9534959
- DOI: 10.1002/advs.202202797
Nanomedicine Strategies for Heating "Cold" Ovarian Cancer (OC): Next Evolution in Immunotherapy of OC
Abstract
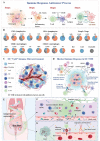
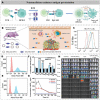
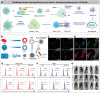
Immunotherapy has revolutionized cancer treatment, dramatically improving survival rates of melanoma and lung cancer patients. Nevertheless, immunotherapy is almost ineffective against ovarian cancer (OC) due to its cold tumor immune microenvironment (TIM). Many traditional medications aimed at remodeling TIM are often associated with severe systemic toxicity, require frequent dosing, and show only modest clinical efficacy. In recent years, emerging nanomedicines have demonstrated extraordinary immunotherapeutic effects for OC by reversing the TIM because the physical and biochemical features of nanomedicines can all be harnessed to obtain optimal and expected tissue distribution and cellular uptake. However, nanomedicines are far from being widely explored in the field of OC immunotherapy due to the lack of appreciation for the professional barriers of nanomedicine and pathology, limiting the horizons of biomedical researchers and materials scientists. Herein, a typical cold tumor-OC is adopted as a paradigm to introduce the classification of TIM, the TIM characteristics of OC, and the advantages of nanomedicines for immunotherapy. Subsequently, current nanomedicines are comprehensively summarized through five general strategies to substantially enhance the efficacy of immunotherapy by heating the cold OC. Finally, the challenges and perspectives of this expanding field for improved development of clinical applications are also discussed.
Keywords: immunotherapy; nanomaterials; nanomedicines; ovarian cancer; tumor immune microenvironment.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., Ca‐Cancer J Clin. 2022, 72, 7. - PubMed
-
- All Cancer Sites Combined Recent Trends in SEER Incidence and U.S. Mortality Rates, 2000–2019, https://seer.cancer.gov/statistics‐network/explorer/application.html (accessed: May 2022).
-
- Pires Da Silva I., Ahmed T., Reijers I. L. M., Weppler A. M., Betof Warner A., Patrinely J. R., Serra‐Bellver P., Allayous C., Mangana J., Nguyen K., Zimmer L., Trojaniello C., Stout D., Lyle M., Klein O., Gerard C. L., Michielin O., Haydon A., Ascierto P. A., Carlino M. S., Lebbe C., Lorigan P., Johnson D. B., Sandhu S., Lo S. N., Blank C. U., Menzies A. M., Long G. V., Lancet Oncol. 2021, 22, 836. - PubMed
-
- Stratigos A. J., Sekulic A., Peris K., Bechter O., Prey S., Kaatz M., Lewis K. D., Basset‐Seguin N., Chang A. L. S., Dalle S. P., Orland A. F., Licitra L., Robert C., Ulrich C., Hauschild A., Migden M. R., Dummer R., Li S., Yoo S.‐Y., Mohan K., Coates E., Jankovic V., Fiaschi N., Okoye E., Bassukas I. D., Loquai C., De Giorgi V., Eroglu Z., Gutzmer R., Ulrich J., et al., Lancet Oncol. 2021, 22, 848. - PubMed
-
- Olino K., Park T., Ahuja N., Semin. Cancer Biol. 2020, 65, 114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
