Neurocomputational mechanisms of confidence in self and others
- PMID: 35869044
- PMCID: PMC9307648
- DOI: 10.1038/s41467-022-31674-w
Neurocomputational mechanisms of confidence in self and others
Abstract
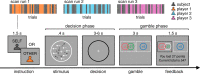
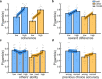
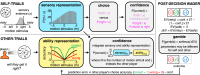
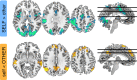
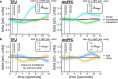
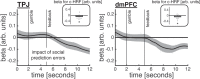
Computing confidence in one's own and others' decisions is critical for social success. While there has been substantial progress in our understanding of confidence estimates about oneself, little is known about how people form confidence estimates about others. Here, we address this question by asking participants undergoing fMRI to place bets on perceptual decisions made by themselves or one of three other players of varying ability. We show that participants compute confidence in another player's decisions by combining distinct estimates of player ability and decision difficulty - allowing them to predict that a good player may get a difficult decision wrong and that a bad player may get an easy decision right. We find that this computation is associated with an interaction between brain systems implicated in decision-making (LIP) and theory of mind (TPJ and dmPFC). These results reveal an interplay between self- and other-related processes during a social confidence computation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

