SMOC2 promotes an epithelial-mesenchymal transition and a pro-metastatic phenotype in epithelial cells of renal cell carcinoma origin
- PMID: 35869056
- PMCID: PMC9307531
- DOI: 10.1038/s41419-022-05059-2
SMOC2 promotes an epithelial-mesenchymal transition and a pro-metastatic phenotype in epithelial cells of renal cell carcinoma origin
Abstract
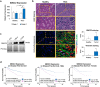
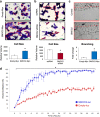
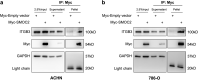
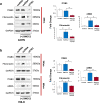
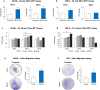
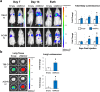
Renal Cell Carcinoma (RCC) is the most common form of all renal cancer cases, and well-known for its highly aggressive metastatic behavior. SMOC2 is a recently described non-structural component of the extracellular matrix (ECM) that is highly expressed during tissue remodeling processes with emerging roles in cancers, yet its role in RCC remains elusive. Using gene expression profiles from patient samples, we identified SMOC2 as being significantly expressed in RCC tissue compared to normal renal tissue, which correlated with shorter RCC patient survival. Specifically, de novo protein synthesis of SMOC2 was shown to be much higher in the tubular epithelial cells of patients with biopsy-proven RCC. More importantly, we provide evidence of SMOC2 triggering kidney epithelial cells into an epithelial-to-mesenchymal transition (EMT), a phenotype known to promote metastasis. We found that SMOC2 induced mesenchymal-like morphology and activities in both RCC and non-RCC kidney epithelial cell lines. Mechanistically, treatment of RCC cell lines ACHN and 786-O with SMOC2 (recombinant and enforced expression) caused a significant increase in EMT-markers, -matrix production, -proliferation, and -migration, which were inhibited by targeting SMOC2 by siRNA. We further characterized SMOC2 activation of EMT to occur through the integrin β3, FAK and paxillin pathway. The proliferation and metastatic potential of SMOC2 overexpressing ACHN and 786-O cell lines were validated in vivo by their significantly higher tumor growth in kidneys and systemic dissemination into other organs when compared to their respective controls. In principle, understanding the impact that SMOC2 has on EMT may lead to more evidence-based treatments and biomarkers for RCC metastasis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Long Non-Coding RNA LOC648987 Promotes Proliferation and Metastasis of Renal Cell Carcinoma by Regulating Epithelial-Mesenchymal Transition.Technol Cancer Res Treat. 2021 Jan-Dec;20:1533033821997834. doi: 10.1177/1533033821997834. Technol Cancer Res Treat. 2021. PMID: 33858283 Free PMC article.
-
Cyclic AMP responsive element-binding protein induces metastatic renal cell carcinoma by mediating the expression of matrix metallopeptidase-2/9 and proteins associated with epithelial-mesenchymal transition.Mol Med Rep. 2017 Jun;15(6):4191-4198. doi: 10.3892/mmr.2017.6519. Epub 2017 Apr 27. Mol Med Rep. 2017. PMID: 28487942
-
LINC00961 restrains cancer progression via modulating epithelial-mesenchymal transition in renal cell carcinoma.J Cell Physiol. 2019 May;234(5):7257-7265. doi: 10.1002/jcp.27483. Epub 2018 Oct 26. J Cell Physiol. 2019. PMID: 30367453
-
The role of hyaluronan in renal cell carcinoma.Front Immunol. 2023 Mar 2;14:1127828. doi: 10.3389/fimmu.2023.1127828. eCollection 2023. Front Immunol. 2023. PMID: 36936902 Free PMC article. Review.
-
Contribution and expression of renal drug transporters in renal cell carcinoma.Front Pharmacol. 2025 Feb 17;15:1466877. doi: 10.3389/fphar.2024.1466877. eCollection 2024. Front Pharmacol. 2025. PMID: 40034145 Free PMC article. Review.
Cited by
-
Role of SMOC2 in adenomyosis: implications for ECM remodeling and EMT pathogenesis.BMC Womens Health. 2025 Apr 3;25(1):155. doi: 10.1186/s12905-025-03700-8. BMC Womens Health. 2025. PMID: 40181364 Free PMC article.
-
nsDCC: dual-level contrastive clustering with nonuniform sampling for scRNA-seq data analysis.Brief Bioinform. 2024 Sep 23;25(6):bbae477. doi: 10.1093/bib/bbae477. Brief Bioinform. 2024. PMID: 39327063 Free PMC article.
-
DERL3 facilitates the progression of clear cell renal cell carcinoma by promoting epithelial-mesenchymal transition via regulation of the TGFB1 pathway.PLoS One. 2025 Apr 29;20(4):e0322172. doi: 10.1371/journal.pone.0322172. eCollection 2025. PLoS One. 2025. PMID: 40299934 Free PMC article.
-
Gene Misexpression in a Smoc2+ve/Sox2-Low Population in Juvenile Prop1-Mutant Pituitary Gland.J Endocr Soc. 2024 Aug 27;8(10):bvae146. doi: 10.1210/jendso/bvae146. eCollection 2024 Aug 27. J Endocr Soc. 2024. PMID: 39253355 Free PMC article.
-
Identification of signature genes and subtypes for heart failure diagnosis based on machine learning.Front Cardiovasc Med. 2025 Apr 14;12:1492192. doi: 10.3389/fcvm.2025.1492192. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40297163 Free PMC article.
References
-
- Canadian Cancer Society., National Cancer Institute of Canada. Advisory Committee on Records and Registries. Canadian cancer statistics. Toronto: Canadian Cancer Society; 1987.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

