Synthetic periphyton as a model system to understand species dynamics in complex microbial freshwater communities
- PMID: 35869094
- PMCID: PMC9307524
- DOI: 10.1038/s41522-022-00322-y
Synthetic periphyton as a model system to understand species dynamics in complex microbial freshwater communities
Abstract
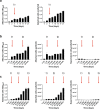
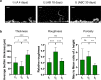
Phototrophic biofilms, also known as periphyton, are microbial freshwater communities that drive crucial ecological processes in streams and lakes. Gaining a deep mechanistic understanding of the biological processes occurring in natural periphyton remains challenging due to the high complexity and variability of such communities. To address this challenge, we rationally developed a workflow to construct a synthetic community by co-culturing 26 phototrophic species (i.e., diatoms, green algae, and cyanobacteria) that were inoculated in a successional sequence to create a periphytic biofilm on glass slides. We show that this community is diverse, stable, and highly reproducible in terms of microbial composition, function, and 3D spatial structure of the biofilm. We also demonstrate the ability to monitor microbial dynamics at the single species level during periphyton development and how their abundances are impacted by stressors such as increased temperature and a herbicide, singly and in combination. Overall, such a synthetic periphyton, grown under controlled conditions, can be used as a model system for theory testing through targeted manipulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wetzel, R. G. Periphyton of Freshwater Ecosystems: Proceedings of the First International Workshop on Periphyton of Freshwater Ecosystems, held in Växjö, Sweden, 14–17 September 1982 (1983).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

