Inhibition of C3 with pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria
- PMID: 35869170
- PMCID: PMC9375762
- DOI: 10.1007/s00277-022-04903-x
Inhibition of C3 with pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria
Abstract
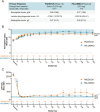
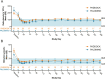
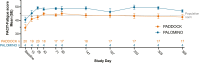
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired hematologic disorder characterized by complement-mediated hemolysis. C5 inhibitors (eculizumab/ravulizumab) control intravascular hemolysis but do not prevent residual extravascular hemolysis. The newly approved complement inhibitor, pegcetacoplan, inhibits C3, upstream of C5, and has the potential to improve control of complement-mediated hemolysis. The PADDOCK and PALOMINO clinical trials assessed the safety and efficacy of pegcetacoplan in complement inhibitor-naïve adults (≥ 18 years) diagnosed with PNH. Patients in PADDOCK (phase 1b open-label, pilot trial) received daily subcutaneous pegcetacoplan (cohort 1: 180 mg up to day 28 [n = 3]; cohort 2: 270-360 mg up to day 365 [n = 20]). PALOMINO (phase 2a, open-label trial) used the same dosing protocol as PADDOCK cohort 2 (n = 4). Primary endpoints in both trials were mean change from baseline in hemoglobin, lactate dehydrogenase, haptoglobin, and the number and severity of treatment-emergent adverse events. Mean baseline hemoglobin levels were below the lower limit of normal in both trials (PADDOCK: 8.38 g/dL; PALOMINO: 7.73 g/dL; normal range: 11.90-18.00 g/dL), increased to within normal range by day 85, and were sustained through day 365 (PADDOCK: 12.14 g/dL; PALOMINO: 13.00 g/dL). In PADDOCK, 3 serious adverse events (SAE) led to study drug discontinuation, 1 of which was deemed likely related to pegcetacoplan and 1 SAE, not deemed related to study drug, led to death. No SAE led to discontinuation/death in PALOMINO. Pegcetacoplan was generally well tolerated and improved hematological parameters by controlling hemolysis, while also improving other clinical PNH indicators in both trials. These trials were registered at www.clinicaltrials.gov (NCT02588833 and NCT03593200).
Keywords: Hemoglobin; LDH; Paroxysmal nocturnal hemoglobinuria (PNH); Phase I/II trials; Quality of life; Safety.
© 2022. The Author(s).
Conflict of interest statement
Raymond S. M. Wong: Alexion: consultancy, honoraria, research funding, and speakers bureau; Apellis: research funding and speakers bureau; Roche: consultancy, honoraria, research funding, and speakers bureau
Humphrey W. H. Pullon: none
Ismail Amine: is a full-time employee of Acibadem City Clinic Tokuda Hospital.
Andrija Bogdanovic: Novartis: consultancy, membership on an entity’s board of directors or advisory committees, and speaker’s bureau; Takeda: membership on an entity’s board of directors or advisory committees, and speaker’s bureau; Pfizer: membership on an entity’s board of directors or advisory committees; Apellis: investigator fee in clinical trial
Pascal Deschatelets is an inventor on patents (planned, issued, or pending) and is the founder and CSO of Apellis Pharmaceuticals.
Cedric G. Francois is an inventor on patents (planned, issued, or pending) and the founder and CEO of Apellis Pharmaceuticals.
Kalina Ignatova: None
Surapol Issaragrisil: Alexion: speakers bureau; Novartis: speakers bureau
Pimjai Niparuck: none
Tontanai Numbenjapon: none
Eloy Roman: Alexion: speakers bureau; Novartis: speakers bureau
Jameela Sathar: Roche: honoraria; Novo Nordisk: honoraria; Bayer: honoraria
Raymond Xu is a full-time employee of Apellis Pharmaceuticals.
Mohammed Al-Adhami is a full-time employee of Apellis Pharmaceuticals.
Lisa Tan as a consultant and is an inventor on patents (planned, issued, or pending) for Apellis Pharmaceuticals.
Eric Tse: MSD: research funding; Janssen: research funding
Federico V. Grossi is an inventor on patents (planned, issued, or pending) and is the CMO of Apellis Pharmaceuticals.
Figures

References
-
- Risitano AM, Notaro R, Marando L, Serio B, Ranaldi D, Seneca E, Ricci P, Alfinito F, Camera A, Gianfaldoni G, Amendola A, Boschetti C, Di Bona E, Fratellanza G, Barbano F, Rodeghiero F, Zanella A, Iori AP, Selleri C, Luzzatto L, Rotoli B. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113(17):4094–4100. doi: 10.1182/blood-2008-11-189944. - DOI - PubMed
-
- Ullman AS, Horn RCJ, Abraham JP, VanSlyck EJ. Paroxysmal nocturnal hemoglobinuria: report of two cases with atypical features, autopsy findings, and review of pathophysiology. Henry Ford Hosp Med Bull. 1963;11(2):135–145. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

