Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria
- PMID: 35869172
- PMCID: PMC9307755
- DOI: 10.1038/s42003-022-03681-6
Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria
Abstract
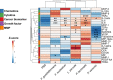
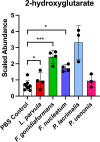
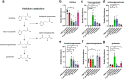
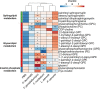
Specific bacteria of the human microbiome influence carcinogenesis at diverse anatomical sites. Bacterial vaginosis (BV) is the most common vaginal disorder in premenopausal women that is associated with gynecologic sequelae, including cervical cancer. BV-associated microorganisms, such as Fusobacterium, Lancefieldella, Peptoniphilus, and Porphyromonas have been associated with gynecologic and other cancers, though the pro-oncogenic mechanisms employed by these bacteria are poorly understood. Here, we integrated a multi-omics approach with our three-dimensional (3-D) cervical epithelial cell culture model to investigate how understudied BV-associated bacteria linked to gynecologic neoplasia influence hallmarks of cancer in vitro. Lancefieldella parvulum and Peptoniphilus lacrimalis elicited robust proinflammatory responses in 3-D cervical cells. Fusobacterium nucleatum and Fusobacterium gonidiaformans modulated metabolic hallmarks of cancer corresponding to accumulation of 2-hydroxyglutarate, pro-inflammatory lipids, and signs of oxidative stress and genotoxic hydrogen sulfide. This study provides mechanistic insights into how gynecologic cancer-associated bacteria might facilitate a tumor-promoting microenvironment in the human cervix.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix.NPJ Biofilms Microbiomes. 2021 Dec 13;7(1):88. doi: 10.1038/s41522-021-00259-8. NPJ Biofilms Microbiomes. 2021. PMID: 34903740 Free PMC article.
-
The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH).APMIS. 2008 Apr;116(4):263-77. doi: 10.1111/j.1600-0463.2008.00808.x. APMIS. 2008. PMID: 18397461
-
Immunometabolic Analysis of Mobiluncus mulieris and Eggerthella sp. Reveals Novel Insights Into Their Pathogenic Contributions to the Hallmarks of Bacterial Vaginosis.Front Cell Infect Microbiol. 2021 Dec 23;11:759697. doi: 10.3389/fcimb.2021.759697. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004344 Free PMC article.
-
Deciphering the Role of Human Gastrointestinal Microbiota in the Pathogenesis of Vaginal Infection and Cervical Cancer.J Environ Pathol Toxicol Oncol. 2020;39(4):365-373. doi: 10.1615/JEnvironPatholToxicolOncol.2020035637. J Environ Pathol Toxicol Oncol. 2020. PMID: 33389908 Review.
-
The Human Microbiome during Bacterial Vaginosis.Clin Microbiol Rev. 2016 Apr;29(2):223-38. doi: 10.1128/CMR.00075-15. Clin Microbiol Rev. 2016. PMID: 26864580 Free PMC article. Review.
Cited by
-
Associations of Atopobium, Garderella, Megasphaera, Prevotella, Sneathia, and Streptococcus with human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis.BMC Infect Dis. 2025 May 16;25(1):708. doi: 10.1186/s12879-025-10851-4. BMC Infect Dis. 2025. PMID: 40380083 Free PMC article.
-
Commensal Lactobacilli Metabolically Contribute to Cervical Epithelial Homeostasis in a Species-Specific Manner.mSphere. 2023 Feb 21;8(1):e0045222. doi: 10.1128/msphere.00452-22. Epub 2023 Jan 11. mSphere. 2023. PMID: 36629413 Free PMC article.
-
Diverse vaginal microbiota in healthy Japanese women: a combined relative and quantitative analyses.Front Cell Infect Microbiol. 2025 Feb 4;14:1487990. doi: 10.3389/fcimb.2024.1487990. eCollection 2024. Front Cell Infect Microbiol. 2025. PMID: 39967801 Free PMC article.
-
Integrated oral-gut microbiota therapy: a novel perspective on preventing bacterial translocation for systemic disease management.Front Cell Infect Microbiol. 2025 Jul 28;15:1641816. doi: 10.3389/fcimb.2025.1641816. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40792109 Free PMC article. Review.
-
The Continuum of Microbial Ecosystems along the Female Reproductive Tract: Implications for Health and Fertility.Pathogens. 2022 Oct 27;11(11):1244. doi: 10.3390/pathogens11111244. Pathogens. 2022. PMID: 36364994 Free PMC article. Review.

