Synthesis of diverse spiro-imidazo pyridine-indene derivatives via acid-promoted annulation reaction of bindone and heterocyclic ketene aminals
- PMID: 35869174
- PMCID: PMC9307797
- DOI: 10.1038/s41598-022-16959-w
Synthesis of diverse spiro-imidazo pyridine-indene derivatives via acid-promoted annulation reaction of bindone and heterocyclic ketene aminals
Abstract
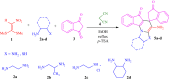
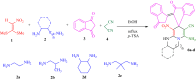
A new multi-component reaction for the synthesis of novel and diverse spiro-imidazo pyridine-indene derivatives named spiro[imidazo[1,2-a]indeno[2,1-e]pyridine-5,1'-indene and indenylidene-1H-spiro[imidazo[1,2-a]pyridine-7,1'-indene was successfully developed by the reaction between heterocyclic ketene aminals (generated from 1,1-bis(methylthio)-2-nitro ethylene and diamine) and [1,2'-biindenylidene]-1',3,3'-trione (bindone) (in situ generated from self-condensation of 1,3-indandion) by using malononitrile as a promoter or as one of the precursors respectively in the presence of p-TSA as the acid catalyst in EtOH as reaction medium under reflux conditions. Depending on whether the reaction is single-step or two-step, malononitrile can act as a promoter or reactant. The convenient one-pot operation, straightforward isolation without using additional purification methods, and the use of a variety of diamines and cysteamine hydrochloride causing a variety of structural products are attractive aspects of the present approach. The synthesized bindone and final product contains active methylene and this active site can be involved in further reactions to synthesize more complex heterocycles.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

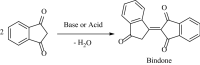




References
-
- Bayat M, Hosseini FS, Notash B. Stereoselective synthesis of indenone-fused heterocyclic compounds via a one-pot four-component reaction. Tetrahedron. 2017;73:1196–1204. doi: 10.1016/j.tet.2017.01.024. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

