Gene regulation by histone-modifying enzymes under hypoxic conditions: a focus on histone methylation and acetylation
- PMID: 35869366
- PMCID: PMC9355978
- DOI: 10.1038/s12276-022-00812-1
Gene regulation by histone-modifying enzymes under hypoxic conditions: a focus on histone methylation and acetylation
Abstract
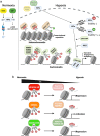
Oxygen, which is necessary for sustaining energy metabolism, is consumed in many biochemical reactions in eukaryotes. When the oxygen supply is insufficient for maintaining multiple homeostatic states at the cellular level, cells are subjected to hypoxic stress. Hypoxia induces adaptive cellular responses mainly through hypoxia-inducible factors (HIFs), which are stabilized and modulate the transcription of various hypoxia-related genes. In addition, many epigenetic regulators, such as DNA methylation, histone modification, histone variants, and adenosine triphosphate-dependent chromatin remodeling factors, play key roles in gene expression. In particular, hypoxic stress influences the activity and gene expression of histone-modifying enzymes, which controls the posttranslational modification of HIFs and histones. This review covers how histone methylation and histone acetylation enzymes modify histone and nonhistone proteins under hypoxic conditions and surveys the impact of epigenetic modifications on gene expression. In addition, future directions in this area are discussed.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

