Trastuzumab therapy duration in HER2-positive de novo metastatic breast cancer: 1999-2018
- PMID: 35869377
- PMCID: PMC9374606
- DOI: 10.1007/s10549-022-06678-1
Trastuzumab therapy duration in HER2-positive de novo metastatic breast cancer: 1999-2018
Abstract
Purpose: The optimal duration of first-line trastuzumab (T) treatment for de novo stage IV HER2-positive metastatic breast cancer (MBC) patients after complete response (CR) is not known.
Methods: A retrospective cohort study of de novo stage IV HER2-positive MBC patients who had trastuzumab included in their initial treatment (n = 69), 1999-2018, was conducted with follow-up for CR, progressive disease (PD), vital status, and disease-specific survival (DSS). Statistics included Kaplan-Meier plots and Cox proportional hazards models.
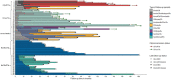
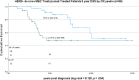
Results: Mean trastuzumab treatment time was 4.1 years (range 0.1-15). 54% of patients experienced CR at average time 9 months on treatment (n = 37). Eight CR patients discontinued T treatment after 18 months average post-CR time (range 0-86) and twenty-nine stayed on T treatment post CR [average 65 months (range 10-170)]. Average follow-up was 6 years, range 1-15 years. 5-year DSS was 92% for CR on T patients (N = 29); 88% CR off T (n = 8); 73% No CR on T (n = 14); and 29% No CR off T (n = 18) (p < 0.001). In forward Cox proportional hazards modeling, CR = yes [HzR = 0.31, (95% CI 0.14, 0.73), p = 0.007], continuous T treatment > 2 years [HzR = 0.24, (95% CI 0.10, 0.62), p = 0.003], and age < 65 [HzR = 0.29, (95% CI 0.11, 0.81), p = 0.018] were significantly associated with better DSS.
Conclusion: Maximum trastuzumab treatment time to CR was 27 months with 2 or more years trastuzumab treatment independently associated with better survival. Survival comparisons and hazard modeling both indicate as good or better survival associated with continuous trastuzumab treatment regardless of CR status. Word count (n = 250).
Keywords: Complete response; HER2; Metastatic breast cancer; Survival; Trastuzumab; Treatment duration.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


Similar articles
-
Patterns of Care and Clinical Outcomes of HER2-positive Metastatic Breast Cancer Patients With Newly Diagnosed Stage IV or Recurrent Disease Undergoing First-line Trastuzumab-based Therapy: A Multicenter Retrospective Cohort Study.Clin Breast Cancer. 2017 Dec;17(8):601-610.e2. doi: 10.1016/j.clbc.2017.04.002. Epub 2017 Apr 11. Clin Breast Cancer. 2017. PMID: 28479052
-
Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990-2010.Breast Cancer Res Treat. 2018 Jan;167(2):579-590. doi: 10.1007/s10549-017-4529-5. Epub 2017 Oct 16. Breast Cancer Res Treat. 2018. PMID: 29039120 Free PMC article.
-
Trastuzumab and survival of patients with metastatic breast cancer.Arch Gynecol Obstet. 2017 Aug;296(2):303-312. doi: 10.1007/s00404-017-4421-x. Epub 2017 Jun 14. Arch Gynecol Obstet. 2017. PMID: 28616827
-
Clinical outcome in women with HER2-positive de novo or recurring stage IV breast cancer receiving trastuzumab-based therapy.Breast. 2014 Feb;23(1):44-9. doi: 10.1016/j.breast.2013.10.005. Epub 2013 Nov 7. Breast. 2014. PMID: 24210572
-
Trastuzumab combined with doublet or single-agent chemotherapy as first-line therapy for HER2-positive metastatic breast cancer.Breast Cancer Res Treat. 2018 Apr;168(2):337-348. doi: 10.1007/s10549-017-4592-y. Epub 2017 Nov 29. Breast Cancer Res Treat. 2018. PMID: 29188398 Free PMC article.
Cited by
-
Time trends in real-world treatment patterns and survival in patients diagnosed with de novo HER2+ metastatic breast cancer: an analysis of the SONABRE registry.Breast Cancer Res Treat. 2024 Jun;205(2):287-302. doi: 10.1007/s10549-023-07235-0. Epub 2024 Feb 21. Breast Cancer Res Treat. 2024. PMID: 38381274 Free PMC article.
-
Protein Kinase Inhibitors as a New Target for Immune System Modulation and Brain Cancer Management.Int J Mol Sci. 2022 Dec 10;23(24):15693. doi: 10.3390/ijms232415693. Int J Mol Sci. 2022. PMID: 36555334 Free PMC article. Review.
References
-
- Wong Y, Raghavendra AS, Hatzis C, Irizarry JP, Vega T, Horowitz N, et al. Long-term survival of de novo stage IV human epidermal growth receptor 2 (HER2) positive breast cancers treated with HER2-targeted therapy. Oncologist. 2019;24(3):313–318. doi: 10.1634/theoncologist.2018-0213. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network. Breast Cancer (Version 2.2022). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Jan 27 2022
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

