Tetrahedral framework nucleic acids promote diabetic wound healing via the Wnt signalling pathway
- PMID: 35869570
- PMCID: PMC9628242
- DOI: 10.1111/cpr.13316
Tetrahedral framework nucleic acids promote diabetic wound healing via the Wnt signalling pathway
Abstract
Objectives: To determine the therapeutic effect of tetrahedral framework nucleic acids (tFNAs) on diabetic wound healing and the underlying mechanism.
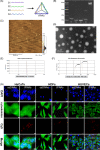
Materials and methods: The tFNAs were characterized by polyacrylamide gel electrophoresis (PAGE), atomic force microscopy (AFM), transmission electron microscopy (TEM), dynamic light scattering (DLS) and zeta potential assays. Cell Counting Kit-8 (CCK-8) and migration assays were performed to evaluate the effects of tFNAs on cellular proliferation and migration. Quantitative polymerase chain reaction (Q-PCR) and enzyme-linked immunosorbent assay (ELISA) were used to detect the effect of tFNAs on growth factors. The function and role of tFNAs in diabetic wound healing were investigated using diabetic wound models, histological analyses and western blotting.
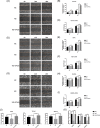
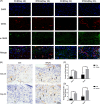
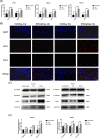
Results: Cellular proliferation and migration were enhanced after treatment with tFNAs in a high-glucose environment. The expression of growth factors was also facilitated by tFNAs in vitro. During in vivo experiments, tFNAs accelerated the healing process in diabetic wounds and promoted the regeneration of the epidermis, capillaries and collagen. Moreover, tFNAs increased the secretion of growth factors and activated the Wnt pathway in diabetic wounds.
Conclusions: This study indicates that tFNAs can accelerate diabetic wound healing and have potential for the treatment of diabetic wounds.
© 2022 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Tetrahedral framework nucleic acids for improving wound healing.J Nanobiotechnology. 2024 Mar 16;22(1):113. doi: 10.1186/s12951-024-02365-z. J Nanobiotechnology. 2024. PMID: 38491372 Free PMC article. Review.
-
Antioxidative and Angiogenesis-Promoting Effects of Tetrahedral Framework Nucleic Acids in Diabetic Wound Healing with Activation of the Akt/Nrf2/HO-1 Pathway.ACS Appl Mater Interfaces. 2020 Mar 11;12(10):11397-11408. doi: 10.1021/acsami.0c00874. Epub 2020 Feb 28. ACS Appl Mater Interfaces. 2020. PMID: 32083455
-
Tetrahedral Framework Nucleic Acids Promote Corneal Epithelial Wound Healing in Vitro and in Vivo.Small. 2019 Aug;15(31):e1901907. doi: 10.1002/smll.201901907. Epub 2019 Jun 13. Small. 2019. PMID: 31192537
-
Tetrahedral framework nucleic acids promote scarless healing of cutaneous wounds via the AKT-signaling pathway.Signal Transduct Target Ther. 2020 Jul 17;5(1):120. doi: 10.1038/s41392-020-0173-3. Signal Transduct Target Ther. 2020. PMID: 32678073 Free PMC article.
-
Recent progress and application of the tetrahedral framework nucleic acid materials on drug delivery.Expert Opin Drug Deliv. 2023 Jul-Dec;20(11):1511-1530. doi: 10.1080/17425247.2023.2276285. Epub 2023 Dec 20. Expert Opin Drug Deliv. 2023. PMID: 37898874 Review.
Cited by
-
Tetrahedral Framework Nucleic Acid Relieves Sepsis-Induced Intestinal Injury by Regulating M2 Macrophages.Cell Prolif. 2025 May;58(5):e13803. doi: 10.1111/cpr.13803. Epub 2025 Jan 22. Cell Prolif. 2025. PMID: 39844345 Free PMC article.
-
Orientin promotes diabetic wounds healing by suppressing ferroptosis via activation of the Nrf2/GPX4 pathway.Food Sci Nutr. 2024 Jul 27;12(10):7461-7480. doi: 10.1002/fsn3.4360. eCollection 2024 Oct. Food Sci Nutr. 2024. PMID: 39479645 Free PMC article.
-
Photoresponsive Multirole Nanoweapon Camouflaged by Hybrid Cell Membrane Vesicles for Efficient Antibacterial Therapy of Pseudomonas aeruginosa-Infected Pneumonia and Wound.Adv Sci (Weinh). 2024 Sep;11(35):e2403101. doi: 10.1002/advs.202403101. Epub 2024 Jul 15. Adv Sci (Weinh). 2024. PMID: 39007186 Free PMC article.
-
Tetrahedral framework nucleic acids for improving wound healing.J Nanobiotechnology. 2024 Mar 16;22(1):113. doi: 10.1186/s12951-024-02365-z. J Nanobiotechnology. 2024. PMID: 38491372 Free PMC article. Review.
-
Exploring the wound healing potential of dietary nitrate in diabetic rat model.Front Physiol. 2024 Nov 20;15:1475375. doi: 10.3389/fphys.2024.1475375. eCollection 2024. Front Physiol. 2024. PMID: 39633648 Free PMC article.
References
-
- Fu J, Huang J, Lin M, Xie T, You T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J Surg Res. 2020;246:213‐223. - PubMed
-
- Fui LW, Lok MPW, Govindasamy V, Yong TK, Lek TK, Das AK. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J Tissue Eng Regen Med. 2019;13(12):2218‐2233. - PubMed
-
- Sanapalli BKR, Yele V, Kalidhindi RSR, Singh SK, Gulati M, Karri VVSR. Human beta defensins may be a multifactorial modulator in the management of diabetic wound. Wound Repair Regen. 2020;28(3):416‐421. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

