Subthalamic nucleus exclusively evokes dopamine release in the tail of the striatum
- PMID: 35869680
- PMCID: PMC9541146
- DOI: 10.1111/jnc.15677
Subthalamic nucleus exclusively evokes dopamine release in the tail of the striatum
Abstract
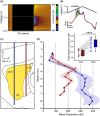
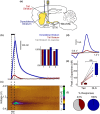
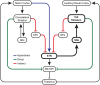
A distinct population of dopamine neurons in the substantia nigra pars lateralis (SNL) has a unique projection to the most caudolateral (tail) region of the striatum. Here, using two electrochemical techniques to measure basal dopamine and electrically evoked dopamine release in anesthetized rats, we characterized this pathway, and compared it with the 'classic' nigrostriatal pathway from neighboring substantia nigra pars compacta (SNc) dopamine neurons to the dorsolateral striatum. We found that the tail striatum constitutes a distinct dopamine domain compared with the dorsolateral striatum, with consistently lower basal and evoked dopamine, and diverse dopamine release kinetics. Importantly, electrical stimulation of the SNL and SNc evoked dopamine release in entirely separate striatal regions; the tail and dorsolateral striatum, respectively. Furthermore, we showed that stimulation of the subthalamic nucleus (STN) evoked dopamine release exclusively in the tail striatum, likely via the SNL, consistent with previous anatomical evidence of STN afferents to SNL dopamine neurons. Our work identifies the STN as an important modulator of dopamine release in a novel dopamine pathway to the tail striatum, largely independent of the classic nigrostriatal pathway, which necessitates a revision of the basal ganglia circuitry with the STN positioned as a central integrator of striatal information.
Keywords: basal ganglia; dopamine; electrochemistry; substantia nigra pars lateralis; subthalamic nucleus; tail striatum.
© 2022 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Albin, R. L. , Young, A. B. , & Penney, J. B. (1989). The functional anatomy of basal ganglia disorders. Trends in Neurosciences, 12, 366–375. - PubMed
-
- Ammari, R. , Lopez, C. , Bioulac, B. , Garcia, L. , & Hammond, C. (2010). Subthalamic nucleus evokes similar long lasting glutamatergic excitations in pallidal, entopeduncular and nigral neurons in the basal ganglia slice. Neuroscience, 166, 808–818. - PubMed
-
- Atcherley, C. W. , Laude, N. D. , Parent, K. L. , & Heien, M. L. (2013). Fast‐scan controlled‐adsorption voltammetry for the quantification of absolute concentrations and adsorption dynamics. Journal of the American Chemical Society, 29, 14885–14892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

