COVID-19 Vaccination in Autoimmune Diseases (COVAD) study: Vaccine safety in idiopathic inflammatory myopathies
- PMID: 35869701
- PMCID: PMC9349921
- DOI: 10.1002/mus.27681
COVID-19 Vaccination in Autoimmune Diseases (COVAD) study: Vaccine safety in idiopathic inflammatory myopathies
Abstract
Introduction/aims: In this study we investigated COVID-19 vaccination-related adverse events (ADEs) 7 days postvaccination in patients with idiopathic inflammatory myopathies (IIMs) and other systemic autoimmune and inflammatory disorders (SAIDs).
Methods: Seven-day vaccine ADEs were collected in an international patient self-reported e-survey. Descriptive statistics were obtained and multivariable regression was performed.
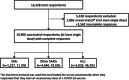
Results: Ten thousand nine hundred respondents were analyzed (1227 IIM cases, 4640 SAID cases, and 5033 healthy controls [HCs]; median age, 42 [interquartile range, 30-455] years; 74% female; 45% Caucasian; 69% completely vaccinated). Major ADEs were reported by 76.3% of the IIM patients and 4.6% reported major ADEs. Patients with active IIMs reported more frequent major (odds ratio [OR], 2.7; interquartile range [IQR], 1.04-7.3) and minor (OR, 1.5; IQR, 1.1-2.2) ADEs than patients with inactive IIMs. Rashes were more frequent in IIMs (OR, 2.3; IQR, 1.2-4.2) than HCs. ADEs were not impacted by steroid dose, although hydroxychloroquine and intravenous/subcutaneous immunoglobulins were associated with a higher risk of minor ADEs (OR, 1.9; IQR, 1.1-3.3; and OR, 2.2; IQR, 1.1-4.3, respectively). Overall, ADEs were less frequent in inclusion-body myositis (IBM) and BNT162b2 (Pfizer) vaccine recipients.
Discussion: Seven-day postvaccination ADEs were comparable in patients with IIMs, SAIDs, and HCs, except for a higher risk of rash in IIMs. Patients with dermatomyositis with active disease may be at higher risk, and IBM patients may be at lower risk of specific ADEs. Overall, the benefit of preventing severe COVID-19 through vaccination likely outweighs the risk of vaccine-related ADEs. Our results may inform future guidelines regarding COVID-19 vaccination in patients with SAIDs, specifically in those with IIMs. Studies to evaluate long-term outcomes and disease flares are needed to shed more light on developing future COVID-19 vaccination guidelines.
Keywords: COVID-19; dermatomyositis; myositis; rheumatology; vaccination.
© 2022 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
J.D. has received research funding from CSL, Ltd. A.L.T. has received honoraria for advisory boards and speaking for AbbVie, Gilead, Janssen, Lilly, Novartis, BNT162b2 (Pfizer), and UCB. E.N. has received speaker honoraria or participated on advisory boards for Celltrion, BNT162b2 (Pfizer), Sanofi, Gilead, Galapagos, AbbVie, and Lilly, and holds research grants from BNT162b2 (Pfizer) and Lilly. H.C. has received grant support from Eli Lilly and UCB, consulting fees from Novartis, Eli Lilly, Orphazyme, and AstraZeneca, and speaker fees from UCB and Biogen. I.P. has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, and Hoffmann‐La Roche. N.Z. has received speaker fees, advisory board fees, and research grants from BNT162b2 (Pfizer), Roche, AbbVie, Eli Lilly, NewBridge, Sanofi‐Aventis, Boehringer Ingelheim, Janssen, and Pierre Fabre (none related to this work). O.D. has/had consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the past 3 years: AbbVie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur, and UCB. O.D. has also issued a patent “mir‐29 for the Treatment of Systemic Sclerosis” (US8247389, EP2331143). R.A. has/had a consultancy relationship with and/or has received research funding from the following companies: Bristol Myers‐Squibb, BNT162b2 (Pfizer), Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, AbbVie, Janssen, Alexion, Argenx, Q32, EMD‐Serono, Boehringer Ingelheim, and Roivant. The remaining authors declare no conflicts of interest.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical