Impact of ischaemic aetiology on the efficacy of intravenous ferric carboxymaltose in patients with iron deficiency and acute heart failure: insights from the AFFIRM-AHF trial
- PMID: 35869741
- PMCID: PMC9826371
- DOI: 10.1002/ejhf.2630
Impact of ischaemic aetiology on the efficacy of intravenous ferric carboxymaltose in patients with iron deficiency and acute heart failure: insights from the AFFIRM-AHF trial
Erratum in
-
Corrigendum to: 'An international Delphi consensus regarding best practice recommendations for hyperkalaemia across the cardiorenal spectrum' and articles listed below.Eur J Heart Fail. 2023 Mar;25(3):444. doi: 10.1002/ejhf.2790. Epub 2023 Feb 17. Eur J Heart Fail. 2023. PMID: 36799255 Free PMC article. No abstract available.
Abstract
Aims: In AFFIRM-AHF, intravenous ferric carboxymaltose (FCM) reduced heart failure (HF) hospitalisations and improved quality of life versus placebo in iron-deficient patients stabilised after an acute HF episode. This analysis explored the effects of FCM versus placebo in patients with ischaemic and non-ischaemic HF aetiology.
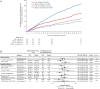
Methods and results: We included 1082 patients from AFFIRM-AHF: 590 with ischaemic HF (defined as investigator-reported ischaemic HF aetiology and/or prior acute myocardial infarction and/or prior coronary revascularisation) and 492 with non-ischaemic HF. The prevalences of male sex, comorbidities, and history of HF were higher in the ischaemic versus non-ischaemic HF subgroup. Annualised event rates for the primary composite outcome of total HF hospitalisations and cardiovascular death with FCM versus placebo were 65.3 versus 100.6 per 100 patient-years in the ischaemic HF subgroup (rate ratio [RR] 0.65, 95% confidence interval [CI] 0.47-0.89, p = 0.007) and 58.3 versus 52.5 in the non-ischaemic HF subgroup (RR 1.11, 95% CI 0.75-1.66, p = 0.60) (pinteraction = 0.039). An interaction between HF aetiology and treatment effect was also observed for the secondary outcome of total HF hospitalisations (pinteraction = 0.038). A nominal increase in quality of life, assessed using the 12-item Kansas City Cardiomyopathy Questionnaire, was observed with FCM versus placebo, within each subgroup.
Conclusions: Heart failure hospitalisations and cardiovascular deaths occurred at a higher rate in patients with ishaemic versus those with non-ischaemic HF and were reduced by FCM versus placebo only in ischaemic patients. Further studies are needed to assess the role of aetiology in FCM efficacy.
Keywords: AFFIRM-AHF; Acute heart failure; Ferric carboxymaltose; Iron deficiency; Ischaemic heart failure.
© 2022 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures



References
-
- Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71:782–93. - PubMed
-
- Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail. 2014;16:984–91. - PubMed
-
- Van Aelst LNL, Abraham M, Sadoune M, Lefebvre T, Manivet P, Logeart D, et al. Iron status and inflammatory biomarkers in patients with acutely decompensated heart failure: early in‐hospital phase and 30‐day follow‐up. Eur J Heart Fail. 2017;19:1075–6. - PubMed
-
- von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7:36–46. - PubMed
-
- Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleskowska‐Florek W, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35:2468–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

