[E.faecium QH06 alleviates TNBS-induced colonic mucosal injury in rats]
- PMID: 35869759
- PMCID: PMC9308865
- DOI: 10.12122/j.issn.1673-4254.2022.07.03
[E.faecium QH06 alleviates TNBS-induced colonic mucosal injury in rats]
Abstract
Objective: To investigate the effect of Enterococcus faecium QH06 on TNBS-induced ulcerative colitis (UC) in rats and explore the mechanisms in light of intestinal flora and intestinal immunity.
Methods: Thirty-six male Wistar rats were randomized equally into control group, UC model group, and E.faecium QH06 intervention group. The rats in the latter two groups were subjected to colonic enema with 5% TNBS/ethanol to induce UC, followed by treatment with intragastric administration of distilled water or E.faecium QH06 at the dose of 0.21 g/kg. After 14 days of treatment, the rats were examined for colon pathologies with HE staining. The mRNA and protein expression levels of IL-4, IL-10, IL-12, and IFN-γ in the colon tissues were detected using RT-qPCR and ELISA, and the expression of TLR2 protein was detected with immunohistochemistry and ELISA. Illumina Miseq platform was used for sequencing analysis of the intestinal flora of the rats with bioinformatics analysis. The correlations of the parameters of the intestinal flora with the expression levels of TLR2 and cytokines were analyzed.
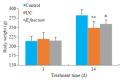
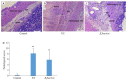
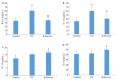
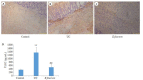
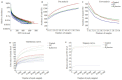
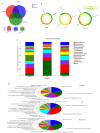
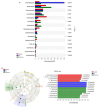
Results: The rats with TNBS- induced UC showed obvious weight loss (P < 0.01) and severe colon tissue injury with high pathological scores (P < 0.01). The protein expression levels of IFN-γ, IL-12, and TLR2 (P < 0.01) and the mRNA expression levels of IFN-γ, IL-12 and IL-10 (P < 0.05) were significantly increased in the colon tissues of the rats with UC. Illumina Miseq sequence analysis showed that in UC rats, the Shannon index (P < 0.05) ACE (P < 0.01)and Chao (P < 0.05) index for the diversity of intestinal flora both decreased with a significantly increased abundance of Enterobacteriaceae (P < 0.01) and a lowered abundance of Burkholderiaceae (P < 0.05). Compared with the UC rats, the rats treated with E. faecium QH06 showed obvious body weight gain (P < 0.05), lessened colon injuries, lowered pathological score of the colon tissue (P < 0.05), decreased protein expressions of IFN- γ, IL- 12, and TLR2 and mRNA expressions of IFN- γ and IL-12 (P < 0.01 or 0.05), and increased protein expressions of IL- 4 (P < 0.05). The Shannon index ACE (P < 0.05) and Chao (P < 0.05) index of intestinal microflora were significantly increased, the abundance of Enterobacteriaceae was lowered and that of Burkholderiaceae and Rikenellaceae was increased in E.faecium QH06- treated rats (P < 0.01 or 0.05). Correlation analysis showed that IFN-γ was positively correlated with the abundance of Enterobacteriaceae, and IFN-γ was negatively correlated with the abundance of Prevotellaceae, Desulfovibrionaceae, norank_o_Mollicutes_RF39 and Clostridiales_vadinBB60_group; TLR2 was negatively correlated with Clostridiales_vadinBB60_group, norank_o_Mollicutes_RF39 and Prevotellaceae.
Conclusion: E.faecium QH06 can alleviate TNBS-induced colonic mucosal injury in rats, and its effect is mediated possibly by increasing the abundance of SCFA-producing bacteria such as Prevotellaceae and inhibiting abnormal immune responses mediated by TLR2.
目的: 探讨屎肠球菌QH06对溃疡性结肠炎(UC)大鼠肠道菌群与肠道免疫的影响及其机制。
方法: 36只SPF级雄性Wistar大鼠随机分为正常对照组、UC模型组和屎肠球菌组(12只/组)。除正常对照组外,其余2组以5% TNBS/乙醇灌肠法建立UC模型。造模成功后屎肠球菌组用屎肠球菌QH06灌胃干预,剂量为0.21 g/kg,正常对照组和UC组用等体积的蒸馏水灌胃干预。干预14 d后用HE染色法观察各组大鼠结肠病理改变并评分,用RT-qPCR和ELISA法检测细胞因子IL-4、IL-10、IL-12和IFN-γ的mRNA和蛋白表达水平,用免疫组化和ELISA法检测TLR2蛋白表达量。利用IlluminaMiseq平台对各组大鼠肠道菌群测序同时进行生物信息学分析。最后肠道菌群和TLR2蛋白表达量、细胞因子等指标进行相关性分析。
结果: 与正常对照组相比,UC组大鼠体质量下降(P < 0.01),结肠组织损伤严重,病理学评分显著升高(P < 0.01);ELISA和RT-qPCR结果显示,IFN-γ(P < 0.01),IL-12(P < 0.01),TLR2(P < 0.01)的蛋白表达水平和IFN-γ(P < 0.05),IL-12(P < 0.05),IL-10(P < 0.05)的mRNA表达水平上调。Illumina Miseq测序和分析显示,UC组大鼠肠道菌群多样性Shannon指数(P < 0.05)、Ace指数(P < 0.01)和Chao指数(P < 0.05)降低。Enterobacteriaceae(P < 0.01)增多,Burkholderiaceae(P < 0.05)减少。与UC组相比,屎肠球菌组大鼠体质量增加(P < 0.05),损伤减轻,病理学评分下降(P < 0.05),结肠组织中IFN-γ(P < 0.01),IL-12(P < 0.05)、TLR2蛋白表达量(P < 0.01)下调,IL-4蛋白表达量上调(P < 0.05),IFN-γ(P < 0.05)、IL-12(P < 0.05)的mRNA表达量下调,肠道菌群多样性Shannon指数(P < 0.05)、Ace指数(P < 0.05)和Chao指数(P < 0.05)升高。Enterobacteriaceae(P < 0.05)减少,Burkholderiaceae(P < 0.05)和Rikenellaceae(P < 0.01)增多;肠道菌群、细胞因子含量和TLR2蛋白表达量相关性分析结果显示,IFN-γ与Enterobacteriaceae呈正相关、而与Prevotellaceae,Desulfovibrionaceae、norank_o__Mollicutes _RF39,Clostridiales _vadinBB60 _group呈负相关;TLR2表达水平与Clostridiales_ vadinBB60_ group,norank_o__Mollicutes_RF39和Prevotellaceae呈负相关。
结论: 屎肠球菌QH06能减轻TNBS诱导的UC大鼠的结肠黏膜损伤,其机制可能与普氏菌科等短链脂肪酸产生菌丰度的升高,TLR2介导的异常免疫反应的抑制有关。
Keywords: Enterococcus faecium; Toll-like receptor 2; cytokines; intestinal flora; ulcerative colitis.
Figures


Similar articles
-
Efficacy of Sishen Wan on dinitrobenzene sulfonic acid-induced ulcerative colitis and its effect on toll-like receptor 2/interleukin-1 receptor-associated kinase-4/nuclear factor-κB signal pathway.J Tradit Chin Med. 2022 Aug;42(4):565-575. doi: 10.19852/j.cnki.jtcm.20220608.001. J Tradit Chin Med. 2022. PMID: 35848973 Free PMC article.
-
[Mechanism of Xiangmei Pills in treating ulcerative colitis based on UHPLC-Q-Orbitrap HRMS and 16S rDNA sequencing of intestinal flora].Zhongguo Zhong Yao Za Zhi. 2025 Feb;50(4):882-895. doi: 10.19540/j.cnki.cjcmm.20240809.401. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40350808 Chinese.
-
[Influence of moxibustion with different duration on colonic epithelial structure, serum inflammatory cytokines, and intestinal mucosa inflammatory cell signal transduction pathways].Zhen Ci Yan Jiu. 2014 Feb;39(1):20-6. Zhen Ci Yan Jiu. 2014. PMID: 24684107 Chinese.
-
[Regulatory effect of Tripterygium wilfordii polycoride (TWP) towards TLR4/MyD88 independent pathway in TNBS/ethanol ulcerative colitis (UC) rat model].Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1093-1099. doi: 10.4268/cjcmm20160620. Zhongguo Zhong Yao Za Zhi. 2016. PMID: 28875676 Chinese.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Progress in the application of Enterococcus faecium in animal husbandry.Front Cell Infect Microbiol. 2023 Jul 28;13:1168189. doi: 10.3389/fcimb.2023.1168189. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37600940 Free PMC article. Review.
-
Bio-Fermented Malic Acid Facilitates the Production of High-Quality Chicken via Enhancing Muscle Antioxidant Capacity of Broilers.Antioxidants (Basel). 2022 Nov 22;11(12):2309. doi: 10.3390/antiox11122309. Antioxidants (Basel). 2022. PMID: 36552518 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
