Neuromuscular mechanisms of weakness in a mouse model of chronic alcoholic myopathy
- PMID: 35869821
- PMCID: PMC9804636
- DOI: 10.1111/acer.14907
Neuromuscular mechanisms of weakness in a mouse model of chronic alcoholic myopathy
Abstract
Background: Weakness is a common clinical symptom reported in individuals with chronic alcohol use disorder. However, it remains unclear whether low strength in these individuals is directly related to excessive ethanol intake, other deleterious factors (lifestyle, environment, genetics, etc.), or a combination of both. Therefore, we examined whether (and how) ethanol reduces the muscle's force-producing capacity using a controlled in vivo preclinical mouse model of excessive ethanol intake.
Methods: To establish whether chronic ethanol consumption causes weakness, C57BL/6 female mice consumed 20% ethanol for 40 weeks (following a 2-week ethanol ramping period), and various measures of muscular force were quantified. Functional measures included all-limb grip strength and in vivo contractility of the left ankle dorsiflexors and plantarflexors. Once confirmed that mice consuming ethanol were weaker than age-matched controls, we sought to determine the potential neuromuscular mechanisms of muscle dysfunction by assessing neuromuscular excitation, muscle quantity, and muscle quality.
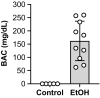
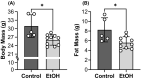
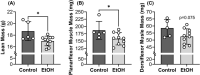
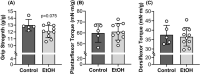
Results: Mice consuming chronic ethanol were 13 to 16% weaker (p ≤ 0.016) than controls (i.e., mice consuming 100% water) with the negative impact of ethanol on voluntary grip strength (ƞ2 = 0.603) being slightly larger than that of electrically stimulated muscle contractility (ƞ2 = 0.482). Relative to controls, lean mass and muscle wet masses were 9 to 16% lower in ethanol-consuming mice (p ≤ 0.048, ƞ2 ≥ 0.268). No significant changes were observed between groups for indices of neuromuscular excitation at the level of the motor unit, neuromuscular junction, or plasmalemma (p ≥ 0.259, ƞ2 ≤ 0.097), nor was muscle quality altered after 40 weeks of 20% ethanol consumption (p ≥ 0.695, ƞ2 ≤ 0.012).
Conclusions: Together, these findings establish that chronic ethanol consumption in mice induces a substantial weakness in vivo that we interpret to be primarily due to muscle atrophy (i.e., reduced muscle quantity) and possibly, to a lesser degree, loss of central neural drive.
Keywords: EtOH; atrophy; force; skeletal muscle; strength.
© 2022 The Authors. Alcoholism: Clinical & Experimental Research published by Wiley Periodicals LLC on behalf of Research Society on Alcoholism.
Conflict of interest statement
In the past 5 years, Brian Clark has received research funding from NMD Pharma, Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., and RTI Health Solutions for contracted studies that involved aging and muscle‐related research. In the past 5 years, Brian Clark has received consulting fees from Regeneron Pharmaceuticals, Zev industries, and the Gerson Lehrman Group for consultation specific to age‐related muscle weakness. Brian Clark is a cofounder with equity of OsteoDx Inc. In the past 5 years, W. David Arnold has received research funding from Biogen, Novartis, Genentech, NMD Pharma, and Avidity Biosciences.
Figures
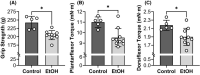

Similar articles
-
Longitudinal assessment of strength and body composition in a mouse model of chronic alcohol-related myopathy.Alcohol Clin Exp Res (Hoboken). 2023 Sep;47(9):1653-1664. doi: 10.1111/acer.15149. Epub 2023 Jul 24. Alcohol Clin Exp Res (Hoboken). 2023. PMID: 37431705
-
Excessive Ethanol Intake in Mice Does Not Impair Recovery of Torque after Repeated Bouts of Eccentric Contractions.Med Sci Sports Exerc. 2023 May 1;55(5):873-883. doi: 10.1249/MSS.0000000000003118. Epub 2023 Jan 5. Med Sci Sports Exerc. 2023. PMID: 36728527
-
Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy.QJM. 2000 Jan;93(1):35-40. doi: 10.1093/qjmed/93.1.35. QJM. 2000. PMID: 10623780
-
Molecular and cellular events in alcohol-induced muscle disease.Alcohol Clin Exp Res. 2007 Dec;31(12):1953-62. doi: 10.1111/j.1530-0277.2007.00530.x. Alcohol Clin Exp Res. 2007. PMID: 18034690 Review.
-
Alcoholic myopathy and acetaldehyde.Novartis Found Symp. 2007;285:158-77; discussion 177-82, 198-9. doi: 10.1002/9780470511848.ch12. Novartis Found Symp. 2007. PMID: 17590994 Review.
Cited by
-
Effects of chronic alcohol intoxication on aerobic exercise-induced adaptations in female mice.J Appl Physiol (1985). 2024 Apr 1;136(4):721-738. doi: 10.1152/japplphysiol.00599.2023. Epub 2024 Feb 15. J Appl Physiol (1985). 2024. PMID: 38357729 Free PMC article.
-
Chronic alcohol-related myopathy: a closer look at the role of lipids.Front Physiol. 2024 Nov 18;15:1492405. doi: 10.3389/fphys.2024.1492405. eCollection 2024. Front Physiol. 2024. PMID: 39624456 Free PMC article.
-
Alcohol and Skeletal Muscle in Health and Disease.Alcohol Res. 2023 Nov 2;43(1):04. doi: 10.35946/arcr.v43.1.04. eCollection 2023. Alcohol Res. 2023. PMID: 37937295 Free PMC article. Review.
-
Role of nutrition in patients with coexisting chronic obstructive pulmonary disease and sarcopenia.Front Nutr. 2023 Aug 8;10:1214684. doi: 10.3389/fnut.2023.1214684. eCollection 2023. Front Nutr. 2023. PMID: 37614743 Free PMC article. Review.
References
-
- Adachi, J. , Asano, M. , Ueno, Y. , Niemelä, O. , Ohlendieck, K. , Peters, T.J. et al. (2003) Alcoholic muscle disease and biomembrane perturbations (review). The Journal of Nutritional Biochemistry, 14, 616–625. - PubMed
-
- Agelink, M.W. , Malessa, R. , Weisser, U. , Lemmer, W. , Zeit, T. , Majewski, T. et al. (1998) Alcoholism, peripheral neuropathy (PNP) and cardiovascular autonomic neuropathy (CAN). Journal of the Neurological Sciences, 161, 135–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

