Polymer Skulls With Integrated Transparent Electrode Arrays for Cortex-Wide Opto-Electrophysiological Recordings
- PMID: 35869830
- PMCID: PMC9573805
- DOI: 10.1002/adhm.202200626
Polymer Skulls With Integrated Transparent Electrode Arrays for Cortex-Wide Opto-Electrophysiological Recordings
Abstract
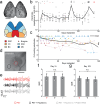
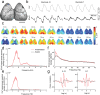
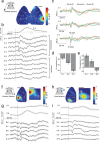
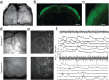
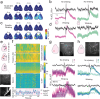
Electrophysiology and optical imaging provide complementary neural sensing capabilities - electrophysiological recordings have high temporal resolution, while optical imaging allows recording of genetically-defined populations at high spatial resolution. Combining these two modalities for simultaneous large-scale, multimodal sensing of neural activity across multiple brain regions can be very powerful. Here, transparent, inkjet-printed electrode arrays with outstanding optical and electrical properties are seamlessly integrated with morphologically conformant transparent polymer skulls. Implanted on transgenic mice expressing the Calcium (Ca2+ ) indicator GCaMP6f in excitatory neurons, these "eSee-Shells" provide a robust opto-electrophysiological interface for over 100 days. eSee-Shells enable simultaneous mesoscale Ca2+ imaging and electrocorticography (ECoG) acquisition from multiple brain regions covering 45 mm2 of cortex under anesthesia and in awake animals. The clarity and transparency of eSee-Shells allow recording single-cell Ca2+ signals directly below the electrodes and interconnects. Simultaneous multimodal measurement of cortical dynamics reveals changes in both ECoG and Ca2+ signals that depend on the behavioral state.
Keywords: calcium imaging; cortex-wide recording; electrophysiology; multi-modal recording; transparent electrodes.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

