Development of an Effective Immune Response in Adults With Down Syndrome After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination
- PMID: 35869848
- PMCID: PMC9384526
- DOI: 10.1093/cid/ciac590
Development of an Effective Immune Response in Adults With Down Syndrome After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination
Erratum in
-
Correction to: Development of an Effective Immune Response in Adults With Down Syndrome After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination.Clin Infect Dis. 2024 Dec 17;79(6):1545. doi: 10.1093/cid/ciae506. Clin Infect Dis. 2024. PMID: 39545819 Free PMC article. No abstract available.
Abstract
Background: Immune dysregulation in individuals with Down syndrome (DS) leads to an increased risk for hospitalization and death due to coronavirus disease 2019 (COVID-19) and may impair the generation of protective immunity after vaccine administration.
Methods: The cellular and humoral responses of 55 individuals with DS who received a complete SARS-CoV-2 vaccination regime at 1 to 3 (visit [V 1]) and 6 (V2) months were characterized.
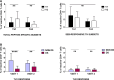
Results: SARS-CoV-2-reactive CD4+ and CD8+ T lymphocytes with a predominant Th1 phenotype were observed at V1 and increased at V2. Likewise, an increase in SARS-CoV-2-specific circulating Tfh (cTfh) cells and CD8+ CXCR5+ PD-1hi lymphocytes was already observed at V1 after vaccine administration. Specific immunoglobulin G (IgG) antibodies against SARS-CoV-2 S protein were detected in 96% and 98% of subjects at V1 and V2, respectively, although IgG titers decreased significantly between both time points.
Conclusions: Our findings show that DS individuals develop an effective immune response to usual regimes of SARS-CoV-2 vaccination.
Keywords: COVID-19; Down syndrome; SARS-CoV-2; immune system; vaccination.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. P.-A. reports fees for Advisory Board participation from Pharmamar and AstraZeneca. D. R. d. A. reports grant number 19/00634 from Instituto Carlos III and grant number 2021A/2069 from Fondation Jerome Lejeune; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Universidad Complutense de Madrid (Título propio de experto en bioética clínica), Weill Cornell Medicine-Qatar (Division of Medical Ethics—several courses), Instituto Universitario de Investigación Ortega Y Gasset (Máster oficial en bioética clínica), Escuela Andaluza de Salud Pública (Curso Consultoría Ética Clínica [2022] y Máster en bioética [2021]), and Consejería de Salud, Comunidad De Madrid (Curso Consultoría Ética Clínica [November 2021]). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

