NPP1 and TNAP hydrolyze ATP synergistically during biomineralization
- PMID: 35870033
- PMCID: PMC10247588
- DOI: 10.1007/s11302-022-09882-2
NPP1 and TNAP hydrolyze ATP synergistically during biomineralization
Abstract
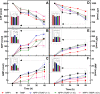
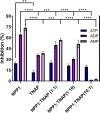
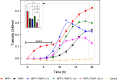
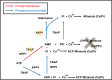
Matrix vesicles (MVs) are a special class of extracellular vesicles released by mineralizing cells during bone and tooth mineralization that initiate the precipitation of apatitic minerals by regulating the extracellular ratio between inorganic phosphate (Pi), a calcification promoter, and pyrophosphate (PPi), a calcification inhibitor. The Pi/PPi ratio is thought to be controlled by two ecto-phosphatases present on the outer leaflet of the MVs' membrane: ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) that produces PPi as well as Pi from ATP and tissue-nonspecific alkaline phosphatase (TNAP) that hydrolyzes both ATP and PPi to generate Pi. However, if and how these enzymes act in concert in MVs are still unclear. Herein, we investigated the role of NPP1 and TNAP in ATP hydrolysis during MV-mediated biomineralization using proteoliposomes as a biomimetic model for MVs. Proteoliposomes composed by 1,2-dipalmitoylphosphatidylcholine (DPPC) and harboring NPP1 alone, TNAP alone, or both together at different molar ratios (1:1, 10:1, and 1:10) were fabricated. After 48 h of incubation with ATP, TNAP-containing proteoliposomes consumed more ATP than NPP1-containing vesicles (270 and 210 nmol, respectively). Both types of vesicles comparatively formed ADP (205 and 201 nmol, respectively), while NPP1-containing vesicles hydrolyzed AMP less efficiently than TNAP-containing proteoliposomes (10 and 25 nmol, respectively). In vitro mineralization assays showed that in the presence of ATP, TNAP-harboring proteoliposomes mineralized through a sigmoidal single-step process, while NPP1-harboring vesicles displayed a two-step mineralization process. ATR-FTIR analyses showed that the minerals produced by TNAP-harboring proteoliposomes were structurally more similar to hydroxyapatite than those produced by NPP1-harboring vesicles. Our results with proteoliposomes indicate that the pyrophosphohydrolase function of NPP1 and the phosphohydrolase activity of TNAP act synergistically to produce a Pi/PPi ratio conducive to mineralization and the synergism is maximal when the two enzymes are present at equimolar concentrations. The significance of these findings for hypophosphatasia is discussed.
Keywords: NPP1; Phosphohydrolase; Proteoliposomes; Pyrophosphohydrolase; TNAP.
© 2022. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Allen MR, Burr DB (2019) Bone growth, modeling, and remodeling. Basic Appl Bone Biol 85–100 10.1016/b978-0-12-813259-3.00005-1
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

