Initiating ivabradine during hospitalization in patients with acute heart failure: A real-world experience in China
- PMID: 35870176
- PMCID: PMC9451666
- DOI: 10.1002/clc.23880
Initiating ivabradine during hospitalization in patients with acute heart failure: A real-world experience in China
Abstract
Background: Initiating ivabradine in acute heart failure (HF) is still controversial.
Hypothesis: Ivabradine might be effective to be added in acute but hemodynamically stable HF.
Methods: A retrospective cohort of hemodynamically stable acute HF patients was enrolled from January 2018 to January 2020 and followed until July 2020. The primary endpoints were all-cause mortality and rehospitalization for HF. Secondary endpoints included heart rate (HR), cardiac function measured by New York Heart Association (NYHA) class, and left ventricular ejection fraction (LVEF) and adverse events, which were compared between patients with or without ivabradine.
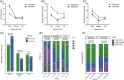
Results: A total of 126 patients were enrolled (50 males, median age 54 years, 81% with decompensated HF, median follow-up of 9 months). In patients treated with ivabradine, although baseline HRs were higher than the reference group (96 vs. 80 bpm), they were comparable after 3 months; more patients tolerated high doses of β-blockers (27% vs. 7.9%), improved to NYHA class I function (55.6% vs. 23.8%) and exhibited normal LVEFs (37.8% vs. 14.3%) than the reference group (all p < .05). Ivabradine was associated with a significant reduction of rehospitalization for HF than the reference group (25.4% vs.61.9%), with longer event-free survival times (hazard ratio: 0.45, 95% confidence interval [CI]: 0.25-0.79), and was related with primary endpoints negatively (hazard ratio 0.51, 95% CI: 0.28-0.91) (all p < .05).
Conclusion: In patients with acute but hemodynamically stable HF, ivabradine may significantly reduce HR, improve cardiac function, and reduce HF rehospitalization.
Keywords: acute heart failure; ivabradine; outcome.
© 2022 The Authors. Clinical Cardiology published by Wiley Periodicals, LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803‐869. - PubMed
-
- Bocchi EA, Salemi VMC. Ivabradine for treatment of heart failure. Expert Opin Drug Saf. 2019;18:393‐402. - PubMed
-
- Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomized placebo‐controlled study. Lancet. 2010;376:875‐885. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2129‐2200. - PubMed
-
- Komajda M, Tavazzi L, Swedberg K, et al. Chronic exposure to ivabradine reduces readmissions in the vulnerable phase after hospitalization for worsening systolic heart failure: a post‐hoc analysis of SHIFT. Eur J Heart Fail. 2016;18:1182‐1189. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

