The hearing hippocampus
- PMID: 35870677
- PMCID: PMC10510040
- DOI: 10.1016/j.pneurobio.2022.102326
The hearing hippocampus
Abstract
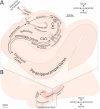
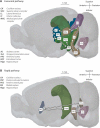
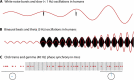
The hippocampus has a well-established role in spatial and episodic memory but a broader function has been proposed including aspects of perception and relational processing. Neural bases of sound analysis have been described in the pathway to auditory cortex, but wider networks supporting auditory cognition are still being established. We review what is known about the role of the hippocampus in processing auditory information, and how the hippocampus itself is shaped by sound. In examining imaging, recording, and lesion studies in species from rodents to humans, we uncover a hierarchy of hippocampal responses to sound including during passive exposure, active listening, and the learning of associations between sounds and other stimuli. We describe how the hippocampus' connectivity and computational architecture allow it to track and manipulate auditory information - whether in the form of speech, music, or environmental, emotional, or phantom sounds. Functional and structural correlates of auditory experience are also identified. The extent of auditory-hippocampal interactions is consistent with the view that the hippocampus makes broad contributions to perception and cognition, beyond spatial and episodic memory. More deeply understanding these interactions may unlock applications including entraining hippocampal rhythms to support cognition, and intervening in links between hearing loss and dementia.
Keywords: Auditory; Auditory cognition; Hearing; Hippocampus; Medial temporal lobe; Perception; Sound.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Abe, R., Sakaguchi, T., Kitajo, K., Ishikawa, D., Matsumoto, N., Matsuki, N., Ikegaya, Y., 2014a. Sound-induced modulation of hippocampal θ oscillations.: NeuroReport 25 2014a 1368 1374 doi: 10.1097/WNR.0000000000000274. - PubMed
-
- Abe, R., Sakaguchi, T., Matsumoto, N., Matsuki, N., Ikegaya, Y., 2014b. Sound-induced hyperpolarization of hippocampal neurons: NeuroReport 25, 1013–1017. https://doi.org/10.1097/WNR.0000000000000206. - PubMed
-
- Abousetta A., Makhlouf N.A., El-Beshbishy R.A. The effects of concomitant Ginkgo intake on noise induced Hippocampus injury. Possible auditory clinical correlate. Egypt. J. Ear Nose Throat Allied Sci. 2014;15:231–239. doi: 10.1016/j.ejenta.2014.05.003. - DOI
-
- Abrams D.A., Chen T., Odriozola P., Cheng K.M., Baker A.E., Padmanabhan A., Ryali S., Kochalka J., Feinstein C., Menon V. Neural circuits underlying mother’s voice perception predict social communication abilities in children. Proc. Natl. Acad. Sci. U.S.A. 2016;113:6295–6300. doi: 10.1073/pnas.1602948113. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

