Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype
- PMID: 35870876
- PMCID: PMC9308124
- DOI: 10.1186/s12879-022-07589-8
Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype
Abstract
Background: The role of ivermectin in the treatment of COVID-19 is still under debate, yet the drug has been widely used in some parts of the world, as shown by impressive market data. The available body of evidence may have changed over the last months, as studies have been retracted and "standards of care" (SOC) used in control groups have changed with rapidly evolving knowledge on COVID-19. This review aims to summarize and critically appraise the evidence of randomized controlled trials (RCTs) of ivermectin, assessing clinical outcomes in COVID-19 patients.
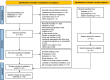
Methods: RCTs evaluating the effects of ivermectin in adult patients with COVID-19 were searched through June 22, 2022, in four databases, L.OVE platform, clinical trial registries and pre-prints platforms. Primary endpoints included all-cause mortality and invasive ventilation requirement. Secondary endpoint was the occurrence of adverse events. Risk of bias was evaluated using the Cochrane Risk of Bias 2.0 tool. Meta-analysis included only studies which compared ivermectin to placebo or SOC. Random-effects were used to pool the risk ratios (RRs) of individual trials. The quality of evidence was evaluated using GRADE. The protocol was register in PROSPERO (CRD42021257471).
Results: Twenty-five RCTs fulfilled inclusion criteria (n = 6310). Of those, 14 compared ivermectin with placebo, in night ivermectin associated with SOC was compared to SOC and two studies compared ivermectin to an active comparator. Most RCTs had some concerns or high risk of bias, mostly due to lack of concealment of the randomization sequence and allocation, lack of blinding and high number of missing cases. Ivermectin did not show an effect in reducing mortality (RR = 0.76; 95%CI: 0.52-1.11) or mechanical ventilation (RR = 0.74; 95%CI: 0.48-1.16). This effect was consistent when comparing ivermectin vs. placebo, and ivermectin associated with SOC vs. SOC, as well as in sensitivity analysis. Additionally, there was very low quality of evidence regarding adverse effects (RR = 1.07; 95%CI: 0.84-1.35).
Conclusions: The evidence suggests that ivermectin does not reduce mortality risk and the risk of mechanical ventilation requirement. Although we did not observe an increase in the risk of adverse effects, the evidence is very uncertain regarding this endpoint.
Keywords: COVID-19; Evidence-based medicine; Ivermectin; Meta-analysis; Mortality; Novel coronavirus; SARS-CoV-2; Systematic review; Therapeutics.
© 2022. The Author(s).
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- World Health Organization. Tracking SARS-CoV-2 variants. World Health Organization International Website. 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants. Accessed 04 Jan 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

