Randomized controlled phase 2 trial of hydroxychloroquine in childhood interstitial lung disease
- PMID: 35871071
- PMCID: PMC9308121
- DOI: 10.1186/s13023-022-02399-2
Randomized controlled phase 2 trial of hydroxychloroquine in childhood interstitial lung disease
Abstract
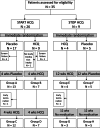
Background: No results of controlled trials are available for any of the few treatments offered to children with interstitial lung diseases (chILD). We evaluated hydroxychloroquine (HCQ) in a phase 2, prospective, multicentre, 1:1-randomized, double-blind, placebo-controlled, parallel-group/crossover trial. HCQ (START arm) or placebo were given for 4 weeks. Then all subjects received HCQ for another 4 weeks. In the STOP arm subjects already taking HCQ were randomized to 12 weeks of HCQ or placebo (= withdrawal of HCQ). Then all subjects stopped treatment and were observed for another 12 weeks.
Results: 26 subjects were included in the START arm, 9 in the STOP arm, of these four subjects participated in both arms. The primary endpoint, presence or absence of a response to treatment, assessed as oxygenation (calculated from a change in transcutaneous O2-saturation of ≥ 5%, respiratory rate ≥ 20% or level of respiratory support), did not differ between placebo and HCQ groups. Secondary endpoints including change of O2-saturation ≥ 3%, health related quality of life, pulmonary function and 6-min-walk-test distance, were not different between groups. Finally combining all placebo and all HCQ treatment periods did not identify significant treatment effects. Overall effect sizes were small. HCQ was well tolerated, adverse events were not different between placebo and HCQ.
Conclusions: Acknowledging important shortcomings of the study, including a small study population, the treatment duration, lack of outcomes like lung function testing below age of 6 years, the small effect size of HCQ treatment observed requires careful reassessments of prescriptions in everyday practice (EudraCT-Nr.: 2013-003714-40, www.clinicaltrialsregister.eu , registered 02.07.2013). Registration The study was registered on 2 July 2013 (Eudra-CT Number: 2013-003714-40), whereas the approval by BfArM was received 24.11.2014, followed by the approval by the lead EC of the University Hospital Munich on 20.01.2015. At clinicaltrials.gov the trial was additionally registered on November 8, 2015 (NCT02615938).
Keywords: Hydroxychloroquine; Interstitial lung diseases; Randomized-controlled trial; chILD.
© 2022. The Author(s).
Conflict of interest statement
The authors do not have competing interests with regard to the study.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical