In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5
- PMID: 35871089
- PMCID: PMC9308029
- DOI: 10.1038/s41598-022-16964-z
In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5
Abstract
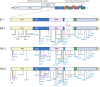
The replacement of the Omicron BA.1 variant of SARS-CoV-2 by the BA.2 and the rapid growth of the BA.5 sub lineage, which have both different sets of mutations in the spike glycoprotein, alters the spectrum of activity of therapeutic antibodies currently licensed in the European Union. Using clinical strains of the Omicron BA.2 and BA.5 variants, we compared the neutralising power of monoclonal antibodies against the Omicron BA.1, BA.2 and BA.5 variants, using an ancestral strain (lineage B.1, D614G) and a Delta variant strain as reference. Sotrovimab/Vir-7831 is less active against BA.2 than against BA.1 (fold change reduction ~ 1,4) and even less active against BA.5 (fold change reduction ~ 2.7). Within the Evusheld /AZD7442 cocktail, Cilgavimab/AZD1061 is more active against BA.2 and BA.5 than against BA.1 (fold change increase ~ 32), whilst the very low activity of Tixagevimab/AZD8895 against BA.1 is not enhanced against BA.2 nor BA.5. In total, compared to BA.1, the activity of the Evusheld/AZD7442 is significantly improved against BA.2 while BA.5 is intermediate but closer to BA.2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

