mRNA- and factor-driven dynamic variability controls eIF4F-cap recognition for translation initiation
- PMID: 35871304
- PMCID: PMC9371892
- DOI: 10.1093/nar/gkac631
mRNA- and factor-driven dynamic variability controls eIF4F-cap recognition for translation initiation
Abstract
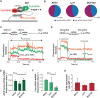
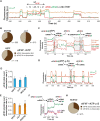
mRNA 5' cap recognition by eIF4F is a key element of eukaryotic translational control. Kinetic differences in eIF4F-mRNA interactions have long been proposed to mediate translation-efficiency differences between mRNAs, and recent transcriptome-wide studies have revealed significant heterogeneity in eIF4F engagement with differentially-translated mRNAs. However, detailed kinetic information exists only for eIF4F interactions with short model RNAs. We developed and applied single-molecule fluorescence approaches to directly observe real-time Saccharomyces cerevisiae eIF4F subunit interactions with full-length polyadenylated mRNAs. We found that eIF4E-mRNA association rates linearly anticorrelate with mRNA length. eIF4G-mRNA interaction accelerates eIF4E-mRNA association in proportion to mRNA length, as does an eIF4F-independent activity of eIF4A, though cap-proximal secondary structure still plays an important role in defining the final association rates. eIF4F-mRNA interactions remained dominated by effects of eIF4G, but were modulated to different extents for different mRNAs by the presence of eIF4A and ATP. We also found that eIF4A-catalyzed ATP hydrolysis ejects eIF4E, and likely eIF4E•eIF4G from the mRNA after initial eIF4F•mRNA complex formation, suggesting a mechanism to prepare the mRNA 5' end for ribosome recruitment. Our results support a role for mRNA-specific, factor-driven eIF4F association rates in kinetically controlling translation.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Yeast eIF4A enhances recruitment of mRNAs regardless of their structural complexity.Elife. 2017 Nov 30;6:e31476. doi: 10.7554/eLife.31476. Elife. 2017. PMID: 29192585 Free PMC article.
-
Human eukaryotic initiation factor 4E (eIF4E) and the nucleotide-bound state of eIF4A regulate eIF4F binding to RNA.J Biol Chem. 2022 Oct;298(10):102368. doi: 10.1016/j.jbc.2022.102368. Epub 2022 Aug 11. J Biol Chem. 2022. PMID: 35963437 Free PMC article.
-
Specific domains in yeast translation initiation factor eIF4G strongly bias RNA unwinding activity of the eIF4F complex toward duplexes with 5'-overhangs.J Biol Chem. 2012 Jun 8;287(24):20301-12. doi: 10.1074/jbc.M112.347278. Epub 2012 Mar 30. J Biol Chem. 2012. PMID: 22467875 Free PMC article.
-
eIF4F: a retrospective.J Biol Chem. 2015 Oct 2;290(40):24091-9. doi: 10.1074/jbc.R115.675280. Epub 2015 Aug 31. J Biol Chem. 2015. PMID: 26324716 Free PMC article. Review.
-
Manipulation of the host translation initiation complex eIF4F by DNA viruses.Biochem Soc Trans. 2010 Dec;38(6):1511-6. doi: 10.1042/BST0381511. Biochem Soc Trans. 2010. PMID: 21118117 Review.
Cited by
-
Single-molecule visualization of mRNA circularization during translation.Exp Mol Med. 2023 Feb;55(2):283-289. doi: 10.1038/s12276-023-00933-1. Epub 2023 Jan 31. Exp Mol Med. 2023. PMID: 36720916 Free PMC article. Review.
-
The mechanism of mRNA activation.bioRxiv [Preprint]. 2023 Nov 15:2023.11.15.567265. doi: 10.1101/2023.11.15.567265. bioRxiv. 2023. Update in: Nature. 2025 Jan;637(8046):736-743. doi: 10.1038/s41586-024-08304-0. PMID: 38014128 Free PMC article. Updated. Preprint.
-
The metaphorical swiss army knife: The multitude and diverse roles of HEAT domains in eukaryotic translation initiation.Nucleic Acids Res. 2022 Jun 10;50(10):5424-5442. doi: 10.1093/nar/gkac342. Nucleic Acids Res. 2022. PMID: 35552740 Free PMC article. Review.
-
The mechanism of mRNA cap recognition.Nature. 2025 Jan;637(8046):736-743. doi: 10.1038/s41586-024-08304-0. Epub 2024 Dec 11. Nature. 2025. PMID: 39663447
-
Quantitative profiling of human translation initiation reveals elements that potently regulate endogenous and therapeutically modified mRNAs.Mol Cell. 2025 Jan 16;85(2):445-459.e5. doi: 10.1016/j.molcel.2024.11.030. Epub 2024 Dec 19. Mol Cell. 2025. PMID: 39706187
References
-
- Marcotrigiano J., Gingras A.C., Sonenberg N., Burley S.K.. Cocrystal structure of the messenger RNA 5’ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell. 1997; 89:951–961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

