ANKLE2-related microcephaly: A variable microcephaly syndrome resembling Zika infection
- PMID: 35871307
- PMCID: PMC9380164
- DOI: 10.1002/acn3.51629
ANKLE2-related microcephaly: A variable microcephaly syndrome resembling Zika infection
Abstract
Objective: This study delineates the clinical and molecular spectrum of ANKLE2-related microcephaly (MIC), as well as highlights shared pathological mechanisms between ANKLE2 and the Zika virus.
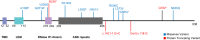
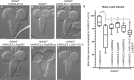
Methods: We identified 12 individuals with MIC and variants in ANKLE2 with a broad range of features. Probands underwent thorough phenotypic evaluations, developmental assessments, and anthropometric measurements. Brain imaging studies were systematically reviewed for developmental abnormalities. We functionally interrogated a subset of identified ANKLE2 variants in Drosophila melanogaster.
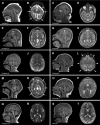
Results: All individuals had MIC (z-score ≤ -3), including nine with congenital MIC. We identified a broad range of brain abnormalities including simplified cortical gyral pattern, full or partial callosal agenesis, increased extra-axial spaces, hypomyelination, cerebellar vermis hypoplasia, and enlarged cisterna magna. All probands had developmental delays in at least one domain, with speech and language delays being the most common. Six probands had skin findings characteristic of ANKLE2 including hyper- and hypopigmented macules. Only one individual had scalp rugae. Functional characterization in Drosophila recapitulated the human MIC phenotype. Of the four variants tested, p.Val229Gly, p.Arg236*, and p.Arg536Cys acted as partial-loss-of-function variants, whereas the c.1421-1G>C splicing variant demonstrated a strong loss-of-function effect.
Interpretation: Deleterious variants in the ANKLE2 gene cause a unique MIC syndrome characterized by congenital or postnatal MIC, a broad range of structural brain abnormalities, and skin pigmentary changes. Thorough functional characterization has identified shared pathogenic mechanisms between ANKLE2-related MIC and congenital Zika virus infection. This study further highlights the importance of a thorough diagnostic evaluation including molecular diagnostic testing in individuals with MIC.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors do not have any real or apparent conflicts of interest.
Figures



References
-
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society . Practice parameter: evaluation of the child with microcephaly (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73(11):887‐897. - PMC - PubMed
-
- Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross‐Sectional Study of the INTERGROWTH‐21st Project. Lancet. 2014;384(9946):857‐868. - PubMed
-
- Morris‐Rosendahl DJ, Kaindl AM. What next‐generation sequencing (NGS) technology has enabled us to learn about primary autosomal recessive microcephaly (MCPH). Mol Cell Probes. 2015;29(5):271‐281. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

