Long-Term Depression-Inducing Low Frequency Stimulation Enhances p-Tau181 and p-Tau217 in an Age-Dependent Manner in Live Rats
- PMID: 35871344
- PMCID: PMC9484260
- DOI: 10.3233/JAD-220351
Long-Term Depression-Inducing Low Frequency Stimulation Enhances p-Tau181 and p-Tau217 in an Age-Dependent Manner in Live Rats
Abstract
Background: Cognitive decline in Alzheimer's disease (AD) correlates with the extent of tau pathology, in particular tau hyperphosphorylation, which is strongly age-associated. Although elevation of cerebrospinal fluid or blood levels of phosphorylated tau (p-Tau) at residues Thr181 (p-Tau181), Thr217 (p-Tau217), and Thr231 (p-Tau231) are proposed to be particularly sensitive markers of preclinical AD, the generation of p-Tau during brain activity is poorly understood.
Objective: To study whether the expression levels of p-Tau181, p-Tau217, and p-Tau231 can be enhanced by physiological synaptic long-term depression (LTD) which has been linked to the enhancement of p-Tau in hippocampus.
Methods: In vivo electrophysiology was performed in urethane anesthetized young adult and aged male rats. Low frequency electrical stimulation (LFS) was used to induce LTD at CA3 to CA1 synapses. The expression level of p-Tau and total tau was measured in dorsal hippocampus using immunofluorescent staining and/or western blotting.
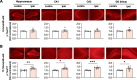
Results: We found that LFS enhanced p-Tau181 and p-Tau217 in an age-dependent manner in the hippocampus of live rats. In contrast, phosphorylation at residues Thr231, Ser202/Thr205, and Ser396 appeared less sensitive to LFS. Pharmacological antagonism of either N-methyl-D-aspartate or metabotropic glutamate 5 receptors inhibited the elevation of both p-Tau181 and p-Tau217. Targeting the integrated stress response, which increases with aging, using a small molecule inhibitor ISRIB, prevented the enhancement of p-Tau by LFS in aged rats.
Conclusion: Together, our data provide a novel in vivo means to uncover brain plasticity-related cellular and molecular processes of tau phosphorylation at key sites in health and aging.
Keywords: Aging; Alzheimer’s disease; integrated stress response; long-term depression; tau phosphorylation.
Conflict of interest statement
Authors’ disclosures available online (
Figures




References
-
- Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, Fatou B, Guise AJ, Cheng L, Takeda S, Muntel J, Rotunno MS, Dujardin S, Davies P, Kosik KS, Miller BL, Berretta S, Hedreen JC, Grinberg LT, Seeley WW, Hyman BT, Steen H, Steen JA (2020) Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell 183, 1699–1713. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

