Topical emollient application in term healthy newborns: A systematic review
- PMID: 35871408
- PMCID: PMC9308984
- DOI: 10.7189/jogh.12.12002
Topical emollient application in term healthy newborns: A systematic review
Abstract
Background: This systematic review of randomized trials assessed the effect of emollient application compared to no emollient application in term or near-term healthy newborns.
Methods: We searched MEDLINE via PubMed, Cochrane CENTRAL, Embase, and CINAHL (updated until November 2021), clinical trials databases, and reference lists of retrieved articles. Key outcomes were neonatal mortality, systemic infections, atopic dermatitis, skin condition, and adverse events. Two authors separately evaluated the risk of bias, extracted data, and synthesized effect estimates using relative risks (RR). The GRADE approach was used to assess the certainty of evidence.
Results: We screened 19 243 records and included 16 eligible trials involving 5643 participants. Five trials recruited 3352 healthy newborns (term = 728; gestation ≥35 weeks = 2624); and 11 trials included 2291 term newborns who were 'at risk' for developing atopy but were otherwise healthy. We conducted a separate analysis for these two groups of newborns. Emollient application (creams or nut, seed, and vegetable oils) started in the neonatal period and continued for four weeks to two years across studies. Meta-analysis for term healthy newborns suggests that topical emollient application may have little to no effect on atopic dermatitis (RR = 1.29, 95% CI = 0.96-1.72; two trials, 1408 newborns; low certainty evidence). Effects on food allergy (RR = 0.84; 95% CI = 0.42-1.70; one trial, 233 newborns), allergic sensitization to food allergens (RR 1.31; 95% CI 1.03 to 1.68; one trial, 234 newborns) and inhalational allergens (RR = 0.97; 95% CI = 0.44, 2.14; 1 trial, 234 newborns), skin dryness (RR = 0.74, 95% CI = 0.55-1.00; two trials, 294 newborns), and skin problems (RR = 0.92, 95% CI = 0.81-1.05; two trials, 292 newborns) were uncertain. Meta-analysis for 'at-risk' newborns suggests that intervention probably lowers the risk of atopic dermatitis (RR = 0.74, 95% CI = 0.63-0.86; 11 studies, 1988 infants; moderate certainty evidence), but may have little or no effect on food allergy and allergic sensitization to food or inhalation allergens. The effect on skin dryness and skin rash was uncertain.
Conclusions: Topical emollient application may not prevent atopic dermatitis in term healthy newborns. There is little data for other skin and allergic outcomes.
Registration: Priyadarshi M, Balachander B, Rao S, Gupta S, Sankar MJ. Use of emollients in term healthy newborns: A systematic review. PROSPERO 2020 CRD42020177437.
Copyright © 2022 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Declaration of interest: The authors completed the ICMJE Unified Competing Interest Form (available upon request from the corresponding author) and declare the following activities and relationships: Shuchita Gupta is a staff member of WHO.
Figures
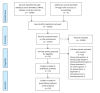
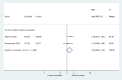
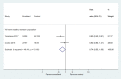


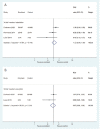
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
