High-performance potassium poly(heptazine imide) films for photoelectrochemical water splitting
- PMID: 35872826
- PMCID: PMC9241972
- DOI: 10.1039/d2sc02043b
High-performance potassium poly(heptazine imide) films for photoelectrochemical water splitting
Abstract
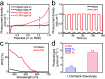
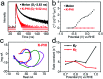
Photoelectrochemical (PEC) water splitting is an appealing approach by which to convert solar energy into hydrogen fuel. Polymeric semiconductors have recently attracted intense interest of many scientists for PEC water splitting. The crystallinity of polymer films is regarded as the main factor that determines the conversion efficiency. Herein, potassium poly(heptazine) imide (K-PHI) films with improved crystallinity were in situ prepared on a conductive substrate as a photoanode for solar-driven water splitting. A remarkable photocurrent density of ca. 0.80 mA cm-2 was achieved under air mass 1.5 global illumination without the use of any sacrificial agent, a performance that is ca. 20 times higher than that of the photoanode in an amorphous state, and higher than those of other related polymeric photoanodes. The boosted performance can be attributed to improved charge transfer, which has been investigated using steady state and operando approaches. This work elucidates the pivotal importance of the crystallinity of conjugated polymer semiconductors for PEC water splitting and other advanced photocatalytic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
One-Pot Synthesis of CoS2 Merged in Polymeric Carbon Nitride Films for Photoelectrochemical Water Splitting.ChemSusChem. 2022 Apr 22;15(8):e202200330. doi: 10.1002/cssc.202200330. Epub 2022 Mar 19. ChemSusChem. 2022. PMID: 35212173
-
BiVO4 Photoanode with Exposed (040) Facets for Enhanced Photoelectrochemical Performance.Nanomicro Lett. 2018;10(1):11. doi: 10.1007/s40820-017-0163-3. Epub 2017 Oct 31. Nanomicro Lett. 2018. PMID: 30393660 Free PMC article.
-
Coating Polymeric Carbon Nitride Photoanodes on Conductive Y:ZnO Nanorod Arrays for Overall Water Splitting.Angew Chem Int Ed Engl. 2018 Jul 26;57(31):9749-9753. doi: 10.1002/anie.201804530. Epub 2018 Jul 4. Angew Chem Int Ed Engl. 2018. PMID: 29901252
-
The Emergence of High-Performance Conjugated Polymer/Inorganic Semiconductor Hybrid Photoelectrodes for Solar-Driven Photoelectrochemical Water Splitting.Small Methods. 2024 Feb;8(2):e2300418. doi: 10.1002/smtd.202300418. Epub 2023 Jul 8. Small Methods. 2024. PMID: 37421184 Review.
-
Recent Advances in TiO2 -based Photoanodes for Photoelectrochemical Water Splitting.Chem Asian J. 2022 Oct 17;17(20):e202200668. doi: 10.1002/asia.202200668. Epub 2022 Sep 8. Chem Asian J. 2022. PMID: 35925726 Review.
Cited by
-
An efficient strategy to boost photoelectrochemical water oxidation of g-C3N4 films modified with NiO as cocatalyst.Sci Rep. 2025 Feb 7;15(1):4632. doi: 10.1038/s41598-025-89031-y. Sci Rep. 2025. PMID: 39920221 Free PMC article.
-
Films of linear conjugated polymer as photoanodes for oxidation reactions.Chem Sci. 2024 Aug 13;15(37):15496-503. doi: 10.1039/d4sc03512g. Online ahead of print. Chem Sci. 2024. PMID: 39246357 Free PMC article.
-
Synergetic effect of photocatalysis and peroxymonosulfate activated by MFe2O4 (M = Co, Mn, or Zn) for enhanced photocatalytic activity under visible light irradiation.RSC Adv. 2022 Jul 21;12(32):20946-20955. doi: 10.1039/d2ra03558h. eCollection 2022 Jul 14. RSC Adv. 2022. PMID: 35919161 Free PMC article.
-
Potassium Poly(heptazine imide) Coupled with Ti3C2 MXene-Derived TiO2 as a Composite Photocatalyst for Efficient Pollutant Degradation.ACS Omega. 2023 Mar 20;8(12):11397-11405. doi: 10.1021/acsomega.3c00150. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008085 Free PMC article.
References
-
- Li X. Wang J. Fang Y. Zhang H. Fu X. Wang X. Acc. Mater. Res. 2021;2:933–943. doi: 10.1021/accountsmr.1c00148. - DOI
LinkOut - more resources
Full Text Sources

