Metabolic Regulation of Cardiac Regeneration
- PMID: 35872916
- PMCID: PMC9304552
- DOI: 10.3389/fcvm.2022.933060
Metabolic Regulation of Cardiac Regeneration
Abstract
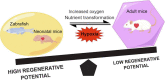
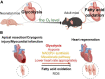
The mortality due to heart diseases remains highest in the world every year, with ischemic cardiomyopathy being the prime cause. The irreversible loss of cardiomyocytes following myocardial injury leads to compromised contractility of the remaining myocardium, adverse cardiac remodeling, and ultimately heart failure. The hearts of adult mammals can hardly regenerate after cardiac injury since adult cardiomyocytes exit the cell cycle. Nonetheless, the hearts of early neonatal mammals possess a stronger capacity for regeneration. To improve the prognosis of patients with heart failure and to find the effective therapeutic strategies for it, it is essential to promote endogenous regeneration of adult mammalian cardiomyocytes. Mitochondrial metabolism maintains normal physiological functions of the heart and compensates for heart failure. In recent decades, the focus is on the changes in myocardial energy metabolism, including glucose, fatty acid, and amino acid metabolism, in cardiac physiological and pathological states. In addition to being a source of energy, metabolites are becoming key regulators of gene expression and epigenetic patterns, which may affect heart regeneration. However, the myocardial energy metabolism during heart regeneration is majorly unknown. This review focuses on the role of energy metabolism in cardiac regeneration, intending to shed light on the strategies for manipulating heart regeneration and promoting heart repair after cardiac injury.
Keywords: amino acid metabolism; cardiomyocyte proliferation; fatty acid metabolism; glucose metabolism; heart regeneration; metabolism regulation.
Copyright © 2022 Duan, Liu and Zhan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

