Fluorescence Imaging of Extracellular Potassium Ion Using Potassium Sensing Oligonucleotide
- PMID: 35873036
- PMCID: PMC9306769
- DOI: 10.3389/fchem.2022.922094
Fluorescence Imaging of Extracellular Potassium Ion Using Potassium Sensing Oligonucleotide
Abstract
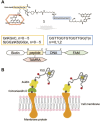
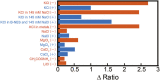
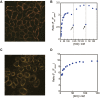
Potassium-sensing oligonucleotide, PSO, a conjugate of a quadruplex structure-forming oligonucleotide with a peptide incorporating a Förster Resonance Energy Transfer (FRET) chromophore pair, has been developed for fluorescent detection of potassium ion (K+) in aqueous medium. PSO 1 could be introduced into cells for real-time imaging of cytoplasmic K+ concentrations. To perform fluorescent imaging of K+ on the cell surface, we synthesized twelve PSO derivatives with different types of peptide types and lengths, and oligonucleotide sequences including thrombin-binding aptamer (TBA) sequences with FAM and TAMRA as a FRET chromophore pair, and evaluated their performance. 1 was shown to respond selectively to K+, not to most ions present in vivo, and to show reciprocal fluorescence changes in response to K+ concentration. For the peptide chains and oligonucleotide sequences examined in this study, the PSO derivatives had K d values for K+ in the range of 5-30 mM. All PSO derivatives showed high K+ selectivity even in the presence of excess Na+. The PSO derivatives were successfully localized to the cell surface by biotinylated concanavalin A (ConA) or sulfo-NHS-biotin via streptavidin (StAv). Fluorescence imaging of extracellular K+ upon addition of apoptosis inducers was successfully achieved by 1 localized to the cell surface.
Keywords: G-quadruplex; cell surface; fluorometric imaging; potassium ion; potassium ion efflux; potassium sensing oligonucleotide; sodium ion.
Copyright © 2022 Sato, Ohzawa, Sota, Sakamoto, Udo, Sueda, Matsuda, Nagai and Takenaka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

