Nanomaterials-Mediated Therapeutics and Diagnosis Strategies for Myocardial Infarction
- PMID: 35873037
- PMCID: PMC9301085
- DOI: 10.3389/fchem.2022.943009
Nanomaterials-Mediated Therapeutics and Diagnosis Strategies for Myocardial Infarction
Abstract
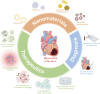
The alarming mortality and morbidity rate of myocardial infarction (MI) is becoming an important impetus in the development of early diagnosis and appropriate therapeutic approaches, which are critical for saving patients' lives and improving post-infarction prognosis. Despite several advances that have been made in the treatment of MI, current strategies are still far from satisfactory. Nanomaterials devote considerable contribution to tackling the drawbacks of conventional therapy of MI by improving the homeostasis in the cardiac microenvironment via targeting, immune modulation, and repairment. This review emphasizes the strategies of nanomaterials-based MI treatment, including cardiac targeting drug delivery, immune-modulation strategy, antioxidants and antiapoptosis strategy, nanomaterials-mediated stem cell therapy, and cardiac tissue engineering. Furthermore, nanomaterials-based diagnosis strategies for MI was presented in term of nanomaterials-based immunoassay and nano-enhanced cardiac imaging. Taken together, although nanomaterials-based strategies for the therapeutics and diagnosis of MI are both promising and challenging, such a strategy still explores the immense potential in the development of the next generation of MI treatment.
Keywords: diagnosis; macrophage; myocardial infarction; nanomaterials; targeted delivery.
Copyright © 2022 Lv, Ma, Li, Fu, Wang and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Biomimetic nanomaterials in myocardial infarction treatment: Harnessing bionic strategies for advanced therapeutics.Mater Today Bio. 2024 Jan 24;25:100957. doi: 10.1016/j.mtbio.2024.100957. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38322664 Free PMC article. Review.
-
Nanomaterials: Promising Tools for the Diagnosis and Treatment of Myocardial Infarction.Int J Nanomedicine. 2025 Feb 11;20:1747-1768. doi: 10.2147/IJN.S500146. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39958320 Free PMC article. Review.
-
Gold Nanoparticle-Based Platforms for Diagnosis and Treatment of Myocardial Infarction.ACS Biomater Sci Eng. 2020 Dec 14;6(12):6460-6477. doi: 10.1021/acsbiomaterials.0c00955. Epub 2020 Nov 17. ACS Biomater Sci Eng. 2020. PMID: 33320615 Review.
-
The clinical prospects and challenges of photothermal nanomaterials in myocardium recovery after myocardial infarction.Front Bioeng Biotechnol. 2024 Oct 29;12:1491581. doi: 10.3389/fbioe.2024.1491581. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39539693 Free PMC article. Review.
-
Nanomaterials as Novel Cardiovascular Theranostics.Pharmaceutics. 2021 Mar 7;13(3):348. doi: 10.3390/pharmaceutics13030348. Pharmaceutics. 2021. PMID: 33799932 Free PMC article. Review.
Cited by
-
Advanced Nanomedicine Approaches for Myocardial Infarction Treatment.Int J Nanomedicine. 2024 Jun 24;19:6399-6425. doi: 10.2147/IJN.S467219. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38952676 Free PMC article. Review.
-
Nanotechnology Innovations in Myocardial Infarction: Diagnosis, Treatment and the Way Forward.J Cardiovasc Transl Res. 2025 Jun;18(3):484-497. doi: 10.1007/s12265-025-10614-1. Epub 2025 Apr 9. J Cardiovasc Transl Res. 2025. PMID: 40205317 Review.
-
Nanozyme-based visual diagnosis and therapeutics for myocardial infarction: The application and strategy.J Adv Res. 2025 Apr;70:187-201. doi: 10.1016/j.jare.2024.04.019. Epub 2024 Apr 23. J Adv Res. 2025. PMID: 38657902 Free PMC article. Review.
-
Research progress on the natural products in the intervention of myocardial infarction.Front Pharmacol. 2024 Aug 22;15:1445349. doi: 10.3389/fphar.2024.1445349. eCollection 2024. Front Pharmacol. 2024. PMID: 39239656 Free PMC article. Review.
-
Editorial: Supramolecular cancer therapeutic biomaterials-volume II.Front Chem. 2025 Mar 5;13:1574334. doi: 10.3389/fchem.2025.1574334. eCollection 2025. Front Chem. 2025. PMID: 40109904 Free PMC article. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

