Novel Synthesis of Sensitive Cu-ZnO Nanorod-Based Sensor for Hydrogen Peroxide Sensing
- PMID: 35873040
- PMCID: PMC9298554
- DOI: 10.3389/fchem.2022.932985
Novel Synthesis of Sensitive Cu-ZnO Nanorod-Based Sensor for Hydrogen Peroxide Sensing
Abstract
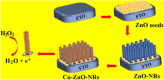
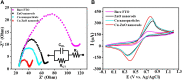
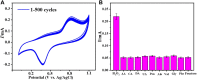
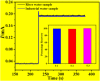
We aimed to synthesize sensitive electrochemical sensors for hydrogen peroxide sensing by using zinc oxide nanorods grown on a fluorine-doped tin oxide electrode by using the facial hydrothermal method. It was essential to keep the surface morphology of the material (nanorods structure); due to its large surface area, the concerned material has enhanced detection ability toward the analyte. The work presents a non-enzymatic H2O2 sensor using vertically grown zinc oxide nanorods on the electrode (FTO) surfaces with Cu nanoparticles deposited on zinc oxide nanorods to enhance the activity. Scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), energy-dispersive X-Ray (EDX), X-ray diffraction (XRD), and electrochemical methods were used to characterize copper-zinc oxide nanorods. In addition to the high surface area, the hexagonal Cu-ZnO nanorods exhibited enhanced electrochemical features of H2O2 oxidation. Nanorods made from Cu-ZnO exhibit highly efficient sensitivity of 3415 μAmM-1cm-2 low detection limits (LODs) of 0.16 μM and extremely wide linear ranges (0.001-11 mM). In addition, copper-zinc oxide nanorods demonstrated decent reproducibility, repeatability, stability, and selectivity after being used for H2O2 sensing in water samples with an RSD value of 3.83%. Cu nanoparticles decorated on ZnO nanorods demonstrate excellent potential for the detection of hydrogen peroxide, providing a new way to prepare hydrogen peroxide detecting devices.
Keywords: Cu-ZnO nanorods; H2O2 detection; electrochemical sensor; hydrothermal method; sensing.
Copyright © 2022 Arsalan, Saddique, Baoji, Awais, Khan, Shamseldin and Mehrez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer DK declared a past co-authorship with the author IK to the handling editor.
Figures





References
-
- Al-Dairy A. R., Albiss B., Jaradat A. A. (2021). Computational Modeling of ZnO-NRs and Graphene Nanostructure as a Glucose Biosensor. Sens. Imaging 22 (1), 1–13. 10.1007/s11220-021-00353-3 - DOI
-
- Arsalan M., Awais A., Chen T., Sheng Q., Zheng J. (2019). Development of PANI/BN-based Absorbents for Water Remediation. Water Qual. Res. J. 54 (4), 290–298. 10.2166/wqrj.2019.048 - DOI
-
- Arsalan M., Awais A., Qiao X., Sheng Q., Zheng J. (2020). Preparation and Comparison of Colloid Based Ni50Co50(OH)2/BOX Electrocatalyst for Catalysis and High Performance Nonenzymatic Glucose Sensor. Microchem. J. 159, 105486. 10.1016/j.microc.2020.105486 - DOI
-
- Arsalan M., Babar N.-U. -A., Sadiqa A., Mansha S., Baig N., Nisar L., et al. (2021). Surface-assembled Fe-Oxide Colloidal Nanoparticles for High Performance Electrocatalytic Water Oxidation. Int. J. Hydrogen Energy 46 (7), 5207–5222. 10.1016/j.ijhydene.2020.11.035 - DOI
LinkOut - more resources
Full Text Sources

