MicroRNA let-7 Suppresses Influenza A Virus Infection by Targeting RPS16 and Enhancing Type I Interferon Response
- PMID: 35873150
- PMCID: PMC9301362
- DOI: 10.3389/fcimb.2022.904775
MicroRNA let-7 Suppresses Influenza A Virus Infection by Targeting RPS16 and Enhancing Type I Interferon Response
Abstract
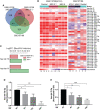
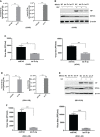
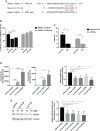
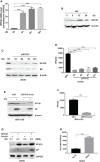
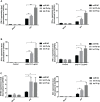
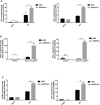
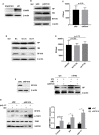
Given the frequent emergence of drug-resistant influenza virus strains and new highly pathogenic influenza virus strains, there is an urgent need to identify new antiviral drugs and targets. We found that influenza A virus (IAV) infection caused a significant decrease of microRNA let-7 expression in host cells; that overexpression of let-7 increased interferon expression and effectively inhibit IAV infection; and that let-7 targets the 3'-untranslated region (UTR) of the ribosomal protein 16 (RPS16) gene, decreasing its expression. Knocking down the expression of RPS16 increased the expression of type I interferon and inhibited viral replication. The present study uncovered the regulatory effect of let-7b and let-7f on influenza A infection, which is a potential biomarker of IAV infection. In addition, let-7 may be a promising therapeutic agent against influenza A.
Keywords: antivirals; influenza A virus; microRNA let-7; rps16; type I interferon.
Copyright © 2022 Wu, Wang, Xia, Liu and Mei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
Similar articles
-
MicroRNA-9-1 Attenuates Influenza A Virus Replication via Targeting Tankyrase 1.J Innate Immun. 2023;15(1):647-664. doi: 10.1159/000532063. Epub 2023 Aug 22. J Innate Immun. 2023. PMID: 37607510 Free PMC article.
-
Dual Effects of Let-7b in the Early Stage of Hepatitis C Virus Infection.J Virol. 2021 Jan 28;95(4):e01800-20. doi: 10.1128/JVI.01800-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33208444 Free PMC article.
-
Influenza A Virus Dysregulates Host Histone Deacetylase 1 That Inhibits Viral Infection in Lung Epithelial Cells.J Virol. 2016 Apr 14;90(9):4614-4625. doi: 10.1128/JVI.00126-16. Print 2016 May. J Virol. 2016. PMID: 26912629 Free PMC article.
-
Host Non-Coding RNA Regulates Influenza A Virus Replication.Viruses. 2021 Dec 29;14(1):51. doi: 10.3390/v14010051. Viruses. 2021. PMID: 35062254 Free PMC article. Review.
-
Targeting the Ubiquitin Proteasome System to Combat Influenza A Virus: Hijacking the Cleanup Crew.Rev Med Virol. 2024 Nov;34(6):e70005. doi: 10.1002/rmv.70005. Rev Med Virol. 2024. PMID: 39516190 Review.
Cited by
-
Porcine Circovirus Modulates Swine Influenza Virus Replication in Pig Tracheal Epithelial Cells and Porcine Alveolar Macrophages.Viruses. 2023 May 20;15(5):1207. doi: 10.3390/v15051207. Viruses. 2023. PMID: 37243291 Free PMC article.
-
The Influence of Extra-Ribosomal Functions of Eukaryotic Ribosomal Proteins on Viral Infection.Biomolecules. 2024 Dec 8;14(12):1565. doi: 10.3390/biom14121565. Biomolecules. 2024. PMID: 39766272 Free PMC article. Review.
-
The Molecular Basis of Asthma Exacerbations Triggered by Viral Infections: The Role of Specific miRNAs.Int J Mol Sci. 2024 Dec 26;26(1):120. doi: 10.3390/ijms26010120. Int J Mol Sci. 2024. PMID: 39795977 Free PMC article. Review.
-
Role of Ocrelizumab in modulating gene and microRNA expression in multiple sclerosis.Noncoding RNA Res. 2025 May 15;13:174-183. doi: 10.1016/j.ncrna.2025.05.009. eCollection 2025 Aug. Noncoding RNA Res. 2025. PMID: 40510879 Free PMC article.
-
Acellular Human Amniotic Fluid-Derived Extracellular Vesicles as Novel Anti-Inflammatory Therapeutics against SARS-CoV-2 Infection.Viruses. 2024 Feb 9;16(2):273. doi: 10.3390/v16020273. Viruses. 2024. PMID: 38400048 Free PMC article.
References
-
- ervantes-Salazar M., Angel-Ambrocio A. H., Soto-Acosta R., Bautista-Carbajal P., Hurtado-Monzon A. M., Alcaraz-Estrada S. L., et al. . (2015). Dengue Virus NS1 Protein Interacts With the Ribosomal Protein RPL18: This Interaction is Required for Viral Translation and Replication in Huh-7 Cells. Virology 484, 113–126. doi: 10.1016/j.virol.2015.05.017 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

