Reduction of 4-nitrophenol using green-fabricated metal nanoparticles
- PMID: 35873318
- PMCID: PMC9228544
- DOI: 10.1039/d2ra02663e
Reduction of 4-nitrophenol using green-fabricated metal nanoparticles
Abstract
Noble metal (silver (Ag), gold (Au), platinum (Pt), and palladium (Pd)) nanoparticles have gained increasing attention due to their importance in several research fields such as environmental and medical research. This review focuses on the basic perceptions of the green synthesis of metal nanoparticles and their supported-catalyst-based reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). The mechanisms for the formation of these nanoparticles and the catalytic reduction of 4-NP are discussed. Furthermore, the parameters that need to be considered in the catalytic efficiency calculations and perspectives for future studies are also discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
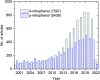
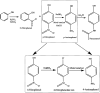
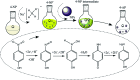


References
-
- United States Environmental Protection Agency, Ambient Water, Quality Criteria for Nitrophenols, 1980
-
- Deka P., Bhattacharjee D., Sarmah P., Deka R. C. and Bharali P., Catalytic Reduction of Water Contaminant ‘4-Nitrophenol’ over Manganese Oxide Supported Ni Nanoparticles, in Trends Asian Water Environ. Sci. Technol., Springer International Publishing, Cham, 2017, pp. 35–48, 10.1007/978-3-319-39259-2_3 - DOI
-
- Neal R. D. Inoue Y. Hughes R. A. Neretina S. Catalytic Reduction of 4-Nitrophenol by Gold Catalysts: The Influence of Borohydride Concentration on the Induction Time. J. Phys. Chem. C. 2019;123:12894–12901. doi: 10.1021/acs.jpcc.9b02396. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

