Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs - a review
- PMID: 35873320
- PMCID: PMC9231466
- DOI: 10.1039/d2ra02896d
Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs - a review
Abstract
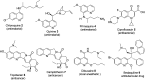
Quinoline, which consists of benzene fused with N-heterocyclic pyridine, has received considerable attention as a core template in drug design because of its broad spectrum of bioactivity. This review aims to present the recent advances in chemistry, medicinal potential and pharmacological applications of quinoline motifs to unveil their substantial efficacies for future drug development. Essential information in all the current and available literature used was accessed and retrieved using different search engines and databases, including Scopus, ISI Web of Knowledge, Google and PUBMED. Numerous derivatives of the bioactive quinolines have been harnessed via expeditious synthetic approaches, as highlighted herein. This review reveals that quinoline is an indisputable pharmacophore due to its tremendous benefits in medicinal chemistry research and other valuable areas of human endeavour. The recent in vivo and in vitro screening reported by scientists is highlighted herein, which may pave the way for novel drug development. Owing to the array of information available and highlighted herein on the medicinal potential of quinoline and its functionalized derivatives, a new window of opportunity may be opened to medicinal chemists to access more biomolecular quinolines for future drug development.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

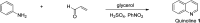
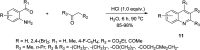
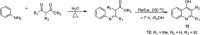
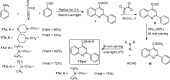

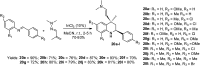
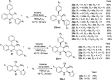

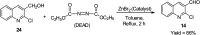
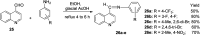
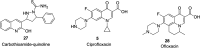
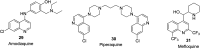
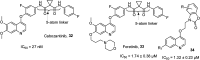

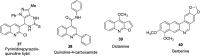
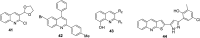
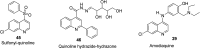


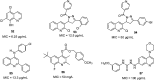




References
-
- Shiro T. Fukaya T. Tobe M. The chemistry and biological activity of heterocycle-fused quinoline derivatives: a review. Eur. J. Med. Chem. 2015;97:397–408. - PubMed
-
- Olateju O. A. Babalola C. P. Olubiyi O. O. Kotila O. A. Kwasi D. A. Oaikhena A. O. Okeke I. N. Quinoline antimalarials increase the antibacterial activity of ampicillin. Front. Microbiol. 2021;12:556550. - PMC - PubMed
- Duparc S. Chalon S. Miller S. Richardson N. Toovey S. Neurological and psychiatric safety of tafenoquine in Plasmodium vivax relapse prevention: a review. Malar. J. 2020;19:111. doi: 10.1186/s12936-020-03184-x. - DOI - PMC - PubMed
- Das R. R. Jaiswal N. Dev N. Jaiswal N. Naik S. S. Shankar J. Efficacy and safety of anti-malarial drugs (chloroquine and hydroxy-chloroquine) in treatment of covid-19 infection: a systematic review and meta-analysis. Front. Med. 2020;7:482. doi: 10.3389/fmed.2020.00482. - DOI - PMC - PubMed
- Sharma V. Mehta D. K. Das R. Synthetic methods of quinoline derivatives as potent anticancer agents. Mini-Rev. Med. Chem. 2017;17(16):1557–1572. - PubMed
-
- Patil V. Barragan E. Patil S. A. Patil S. A. Bugarin A. Direct synthesis and antimicrobial evaluation of structurally complex chalcones. ChemistrySelect. 2016;1(13):3647–3650.
Publication types
LinkOut - more resources
Full Text Sources

