Heterocycles from cyclopropenones
- PMID: 35873324
- PMCID: PMC9229296
- DOI: 10.1039/d2ra03011j
Heterocycles from cyclopropenones
Abstract
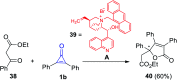
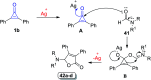
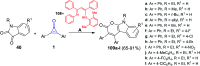
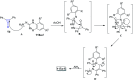
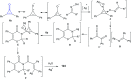
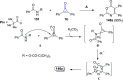
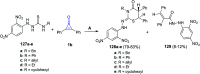
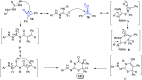
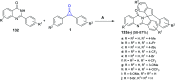
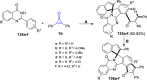
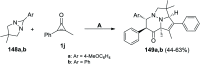
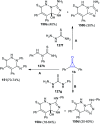
Great attention has been paid to cyclopropenones as they are present in many natural sources. Various synthesized cyclopropenone derivatives also show a wide range of biological activities. The cyclopropenone derivatives undergo a variety of reactions such as ring-opening reactions, isomerization reactions, C-C coupling reactions, C-H activation, cycloaddition reactions, thermal and photo-irradiation reactions, and acid-base-catalyzed reactions under the influence of various chemical reagents (electrophiles, nucleophiles, radicals, and organometallics) and external forces (heat and light). Many previous reviews have dealt with the chemistry and reactions of cyclopropenones. However and to the best of our knowledge, the utility of cyclopropenones in the synthesis of heterocycles has not been reported before. Therefore, it would be interesting to shed light on this new topic. The present review article provides, for the first time, a comprehensive compilation of synthetic methods for the synthesis of various heterocyclic ring systems, as a significant family in the field of organic chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

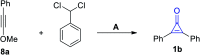
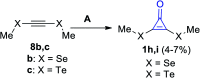
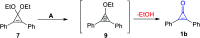
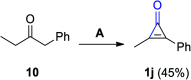
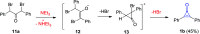
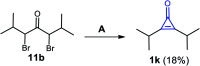
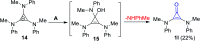
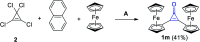
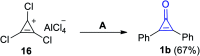
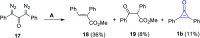
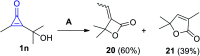
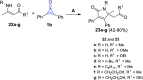
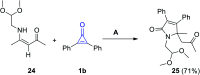
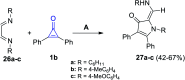
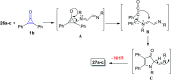
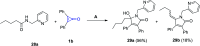
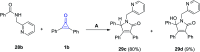
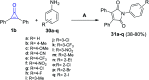
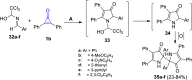
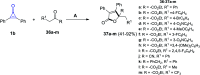
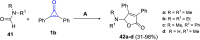

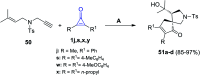
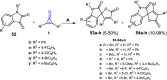
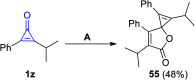
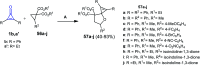
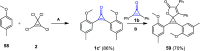
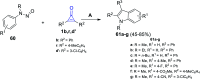
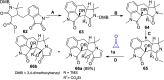
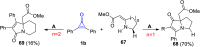
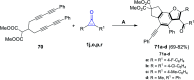
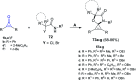
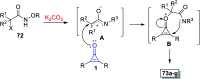
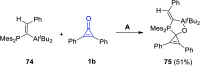

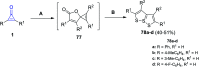
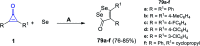
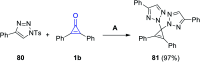
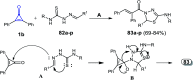
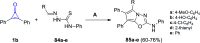
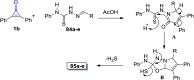
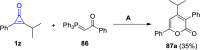
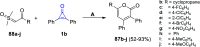
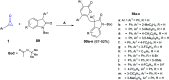
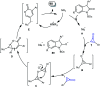
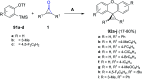
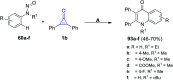
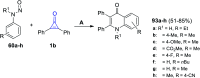
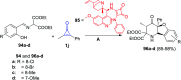
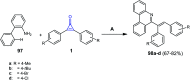
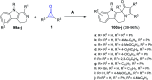
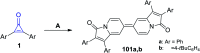
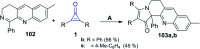
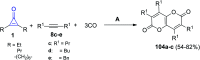
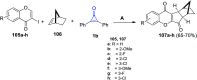
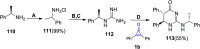
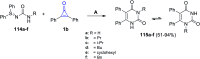


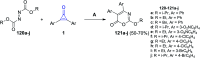
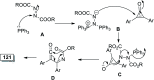
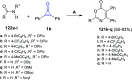
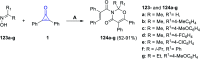
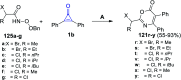
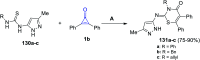
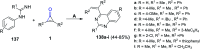
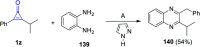
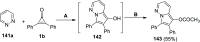
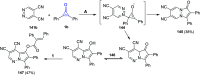
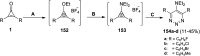
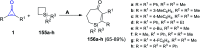
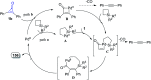
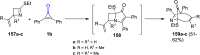
References
-
- Dong G., C-C Bond Activation, Springer, Berlin, 1st edn, 2014, vol. 346, pp. 1–258
-
- Jones W. D. Nature. 1993;364:676–677. doi: 10.1038/364676a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

