Necroptosis-Mediated eCIRP Release in Sepsis
- PMID: 35873387
- PMCID: PMC9304637
- DOI: 10.2147/JIR.S370615
Necroptosis-Mediated eCIRP Release in Sepsis
Erratum in
-
Erratum: Necroptosis-Mediated eCIRP Release in Sepsis [Corrigendum].J Inflamm Res. 2022 Aug 1;15:4347-4348. doi: 10.2147/JIR.S383613. eCollection 2022. J Inflamm Res. 2022. PMID: 35937917 Free PMC article.
Abstract
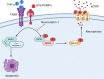
Introduction: Extracellular cold-inducible RNA-binding protein (eCIRP) is an endogenous pro-inflammatory mediator that exacerbates injury in inflammation and sepsis. The mechanisms in which eCIRP is released have yet to be fully explored. Necroptosis is a programmed cell death that is dependent on the activation of mixed lineage kinase domain-like pseudo kinase (MLKL) which causes the release of damage-associated molecular patterns. We hypothesize that eCIRP is released through necroptosis and intensifies inflammation in sepsis.
Methods: RAW264.7 cells were treated with pan-caspase inhibitor z-VAD (15 μM) 1 h before stimulation with LPS (1 μg/mL). Necroptosis inhibitor, Necrostatin-1 (Nec-1) (10 μM) was added to the cells with LPS simultaneously. After 24 h of LPS stimulation, cytotoxicity was determined by LDH assay. eCIRP levels in the culture supernatants and phospho-MLKL (p-MLKL) from cell lysates were assessed by Western blot. p-MLKL interaction with the cell membrane was visualized by immunofluorescence. Sepsis was induced in C57BL/6 mice by cecal ligation and puncture (CLP). Mice were treated with Nec-1 (1 mg/kg) or DMSO. 20 h post-surgery, serum and peritoneal fluid levels of eCIRP, TNF-α and IL-6 were determined by ELISA. H&E staining of lung tissue sections was performed.
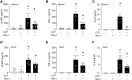
Results: We found that in RAW264.7 cells, LPS+z-VAD induces necroptosis as evidenced by an increase in p-MLKL levels and causes eCIRP release. Nec-1 reduces both p-MLKL activation and eCIRP release in LPS+z-VAD-treated RAW264.7 cells. Nec-1 also inhibits the release of eCIRP, TNF-α and IL-6 in the serum and peritoneal fluid in CLP-induced septic mice. We predicted a transient interaction between eCIRP and MLKL using a computational model, suggesting that eCIRP may exit the cell via the pores formed by p-MLKL.
Conclusion: Necroptosis is a novel mechanism of eCIRP release in sepsis. Targeting necroptosis may ameliorate inflammation and injury in sepsis by inhibiting eCIRP release.
Keywords: Necrostatin-1; eCIRP; macrophage; necroptosis; sepsis.
© 2022 Reilly et al.
Conflict of interest statement
The authors declared that they have no conflicts of interest in relation to this work.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

