Strongly Truncated Dnaaf4 Plays a Conserved Role in Drosophila Ciliary Dynein Assembly as Part of an R2TP-Like Co-Chaperone Complex With Dnaaf6
- PMID: 35873488
- PMCID: PMC9298768
- DOI: 10.3389/fgene.2022.943197
Strongly Truncated Dnaaf4 Plays a Conserved Role in Drosophila Ciliary Dynein Assembly as Part of an R2TP-Like Co-Chaperone Complex With Dnaaf6
Abstract
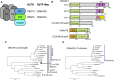
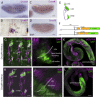
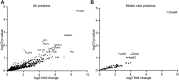
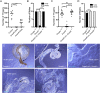
Axonemal dynein motors are large multi-subunit complexes that drive ciliary movement. Cytoplasmic assembly of these motor complexes involves several co-chaperones, some of which are related to the R2TP co-chaperone complex. Mutations of these genes in humans cause the motile ciliopathy, Primary Ciliary Dyskinesia (PCD), but their different roles are not completely known. Two such dynein (axonemal) assembly factors (DNAAFs) that are thought to function together in an R2TP-like complex are DNAAF4 (DYX1C1) and DNAAF6 (PIH1D3). Here we investigate the Drosophila homologues, CG14921/Dnaaf4 and CG5048/Dnaaf6. Surprisingly, Drosophila Dnaaf4 is truncated such that it completely lacks a TPR domain, which in human DNAAF4 is likely required to recruit HSP90. Despite this, we provide evidence that Drosophila Dnaaf4 and Dnaaf6 proteins can associate in an R2TP-like complex that has a conserved role in dynein assembly. Both are specifically expressed and required during the development of the two Drosophila cell types with motile cilia: mechanosensory chordotonal neurons and sperm. Flies that lack Dnaaf4 or Dnaaf6 genes are viable but with impaired chordotonal neuron function and lack motile sperm. We provide molecular evidence that Dnaaf4 and Dnaaf6 are required for assembly of outer dynein arms (ODAs) and a subset of inner dynein arms (IDAs).
Keywords: Drosophila; chaperone; ciliopathies; cilium; dynein; flagellum.
Copyright © 2022 Lennon, zur Lage, von Kriegsheim and Jarman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility.PLoS Genet. 2021 Feb 26;17(2):e1009306. doi: 10.1371/journal.pgen.1009306. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33635866 Free PMC article.
-
X-linked primary ciliary dyskinesia due to mutations in the cytoplasmic axonemal dynein assembly factor PIH1D3.Nat Commun. 2017 Feb 8;8:14279. doi: 10.1038/ncomms14279. Nat Commun. 2017. PMID: 28176794 Free PMC article.
-
Survey of the Ciliary Motility Machinery of Drosophila Sperm and Ciliated Mechanosensory Neurons Reveals Unexpected Cell-Type Specific Variations: A Model for Motile Ciliopathies.Front Genet. 2019 Feb 1;10:24. doi: 10.3389/fgene.2019.00024. eCollection 2019. Front Genet. 2019. PMID: 30774648 Free PMC article.
-
Role of the Novel Hsp90 Co-Chaperones in Dynein Arms' Preassembly.Int J Mol Sci. 2019 Dec 7;20(24):6174. doi: 10.3390/ijms20246174. Int J Mol Sci. 2019. PMID: 31817850 Free PMC article. Review.
-
Primary Ciliary Dyskinesia.2007 Jan 24 [updated 2025 May 22]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2007 Jan 24 [updated 2025 May 22]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301301 Free Books & Documents. Review.
Cited by
-
Transcriptional and Translational Regulation of Differentially Expressed Genes in Yucatan Miniswine Brain Tissues following Traumatic Brain Injury.J Bioinform Syst Biol. 2024;7(1):81-91. doi: 10.26502/jbsb.5107080. Epub 2024 Mar 5. J Bioinform Syst Biol. 2024. PMID: 38818113 Free PMC article.
-
PIH1D3-knockout rats exhibit full ciliopathy features and dysfunctional pre-assembly and loading of dynein arms in motile cilia.Front Cell Dev Biol. 2023 Oct 12;11:1282787. doi: 10.3389/fcell.2023.1282787. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900281 Free PMC article.
-
Schmidtea mediterranea as a Model Organism to Study the Molecular Background of Human Motile Ciliopathies.Int J Mol Sci. 2023 Feb 24;24(5):4472. doi: 10.3390/ijms24054472. Int J Mol Sci. 2023. PMID: 36901899 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

