The Neuroprotective Role of Retbindin, a Metabolic Regulator in the Neural Retina
- PMID: 35873559
- PMCID: PMC9298789
- DOI: 10.3389/fphar.2022.919667
The Neuroprotective Role of Retbindin, a Metabolic Regulator in the Neural Retina
Abstract
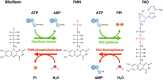
Dysregulation of retinal metabolism is emerging as one of the major reasons for many inherited retinal diseases (IRDs), a leading cause of blindness worldwide. Thus, the identification of a common regulator that can preserve or revert the metabolic ecosystem to homeostasis is a key step in developing a treatment for different forms of IRDs. Riboflavin (RF) and its derivatives (flavins), flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), are essential cofactors for a wide range of cellular metabolic processes; hence, they are particularly critical in highly metabolically active tissues such as the retina. Patients with RF deficiency (ariboflavinosis) often display poor photosensitivity resulting in impaired low-light vision. We have identified a novel retina-specific RF binding protein called retbindin (Rtbdn), which plays a key role in retaining flavin levels in the neural retina. This role is mediated by its specific localization at the interface between the neural retina and retinal pigment epithelium (RPE), which is essential for metabolite and nutrient exchange. As a consequence of this vital function, Rtbdn's role in flavin utilization and metabolism in retinal degeneration is discussed. The principal findings are that Rtbdn helps maintain high levels of retinal flavins, and its ablation leads to an early-onset retinal metabolic dysregulation, followed by progressive degeneration of rod and cone photoreceptors. Lack of Rtbdn reduces flavin levels, forcing the neural retina to repurpose glucose to reduce the production of free radicals during ATP production. This leads to metabolic breakdown followed by retinal degeneration. Assessment of the role of Rtbdn in several preclinical retinal disease models revealed upregulation of its levels by several folds prior to and during the degenerative process. Ablation of Rtbdn in these models accelerated the rate of retinal degeneration. In agreement with these in vivo studies, we have also demonstrated that Rtbdn protects immortalized cone photoreceptor cells (661W cells) from light damage in vitro. This indicates that Rtbdn plays a neuroprotective role during retinal degeneration. Herein, we discussed the specific function of Rtbdn and its neuroprotective role in retinal metabolic homeostasis and its role in maintaining retinal health.
Keywords: flavins; neuroprotection; retbindin; retinal metabolism; retinal regeneration; riboflavin.
Copyright © 2022 Zhao, Tebbe, Naash and Al-Ubaidi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Batey D. W., Eckhert C. D. (1991). Analysis of Flavins in Ocular Tissues of the Rabbit. Invest. Ophthalmol. Vis. Sci. 32, 1981–1985. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

