Efficacy and Safety of a Botanical Formula Fuzheng Huayu for Hepatic Fibrosis in Patients with CHC: Results of a Phase 2 Clinical Trial
- PMID: 35873630
- PMCID: PMC9307334
- DOI: 10.1155/2022/4494099
Efficacy and Safety of a Botanical Formula Fuzheng Huayu for Hepatic Fibrosis in Patients with CHC: Results of a Phase 2 Clinical Trial
Abstract
Background: Hepatitis C virus (HCV) is a common cause of progressive hepatic fibrosis, cirrhosis, and hepatocellular carcinoma worldwide. Despite the availability of effective direct-acting antivirals, patients often have significant hepatic fibrosis at the time of diagnosis due to delay in diagnosis and comorbidities which promote fibrogenesis. Thus, antifibrotic agents represent an attractive adjunctive therapy. Fuzheng Huayu (FZHY), a traditional Chinese medicine botanical formulation, has been used as an antifibrotic agent in chronic HBV infection. Our aim was to assess FZHY in patients with HCV infection and active viremia.
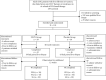
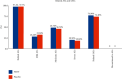
Method: We randomized 118 patients with active viremia from 8 liver centers in the U.S. to receive oral FZHY (n = 59) or placebo (n = 59) for 48 weeks. Efficacy was assessed by histopathologic changes at the end of therapy. A subset of biopsies was further analyzed using qFibrosis to detect subtle changes in fibrosis in different zones of the hepatic lobules.
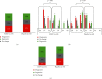
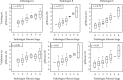
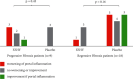
Results: FZHY was well tolerated and safe. Patients with baseline Ishak fibrosis stages F3 and F4 had better response rates to FZHY than patients with baseline F0-F2 (p=0.03). qFibrosis zonal analysis showed significant improvement in fibrosis in all zones in patients with regression of the fibrosis stage.
Conclusions: FZHY produced antifibrotic effects in patients with baseline Ishak F3 and F4 fibrosis stages. Reduction in fibrosis severity was zonal and correlated with the severity of inflammation. Based on its tolerability, safety, and efficacy, FZHY should be further investigated as a therapy in chronic liver diseases because of its dual anti-inflammatory and antiibrotic properties. Lay Summary. This is the first US-based, multicenter and placebo-controlled clinical trial that shows statistically significant reduction in fibrosis in patients with active HCV using an antifibrotic botanical formula. This has important implications as there is an immediate need for effective antifibrotic agents in treating many chronic diseases including NASH that lead to scarring of the liver. With artificial intelligence-based methodology, qFibrosis, we may provide a more reliable way to assess the FZHY as a therapy in chronic liver diseases because of its dual anti-inflammatory and antifibrotic properties.
Copyright © 2022 Tarek Hassanein et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Petruzziello A., Marigliano S., Loquercio G., Cozzolino A., Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World Journal of Gastroenterology . 2016;22(34):7824–7840. doi: 10.3748/wjg.v22.i34.7824. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

