Chlorpromazine and Promethazine (C+P) Reduce Brain Injury after Ischemic Stroke through the PKC- δ/NOX/MnSOD Pathway
- PMID: 35873710
- PMCID: PMC9307415
- DOI: 10.1155/2022/6886752
Chlorpromazine and Promethazine (C+P) Reduce Brain Injury after Ischemic Stroke through the PKC- δ/NOX/MnSOD Pathway
Abstract
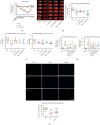
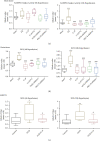
Cerebral ischemia-reperfusion (I/R) incites neurologic damage through a myriad of complex pathophysiological mechanisms, most notably, inflammation and oxidative stress. In I/R injury, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) produces reactive oxygen species (ROS), which promote inflammatory and apoptotic pathways, augmenting ROS production and promoting cell death. Inhibiting ischemia-induced oxidative stress would be beneficial for reducing neuroinflammation and promoting neuronal cell survival. Studies have demonstrated that chlorpromazine and promethazine (C+P) induce neuroprotection. This study investigated how C+P minimizes oxidative stress triggered by ischemic injury. Adult male Sprague-Dawley rats were subject to middle cerebral artery occlusion (MCAO) and subsequent reperfusion. 8 mg/kg of C+P was injected into the rats when reperfusion was initiated. Neurologic damage was evaluated using infarct volumes, neurological deficit scoring, and TUNEL assays. NOX enzymatic activity, ROS production, protein expression of NOX subunits, manganese superoxide dismutase (MnSOD), and phosphorylation of PKC-δ were assessed. Neural SHSY5Y cells underwent oxygen-glucose deprivation (OGD) and subsequent reoxygenation and C+P treatment. We also evaluated ROS levels and NOX protein subunit expression, MnSOD, and p-PKC-δ/PKC-δ. Additionally, we measured PKC-δ membrane translocation and the level of interaction between NOX subunit (p47phox) and PKC-δ via coimmunoprecipitation. As hypothesized, treatment with C+P therapy decreased levels of neurologic damage. ROS production, NOX subunit expression, NOX activity, and p-PKC-δ/PKC-δ were all significantly decreased in subjects treated with C+P. C+P decreased membrane translocation of PKC-δ and lowered the level of interaction between p47phox and PKC-δ. This study suggests that C+P induces neuroprotective effects in ischemic stroke through inhibiting oxidative stress. Our findings also indicate that PKC-δ, NOX, and MnSOD are vital regulators of oxidative processes, suggesting that C+P may serve as an antioxidant.
Copyright © 2022 Sichao Guo et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

