Flavonoid Accumulation Varies in Medicago truncatula in Response to Mercury Stress
- PMID: 35874019
- PMCID: PMC9301243
- DOI: 10.3389/fpls.2022.933209
Flavonoid Accumulation Varies in Medicago truncatula in Response to Mercury Stress
Abstract
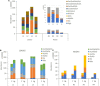
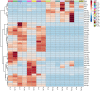
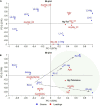
Mercury (Hg) contamination is increasing worldwide in both wild ecosystems and agricultural soils due to natural processes, but mostly to anthropic activities. The molecular mechanisms involved in Hg toxicity and tolerance in plants have been extensively studied; however, the role of flavonoids in response to Hg stress remains to be investigated. We conducted a metabolomic study to analyze the changes induced at the secondary metabolite level in three Hg-tolerant and one Hg-sensitive Medicago truncatula cultivars. A total of 46 flavonoid compounds, classified into five different flavonoid families: anthocyanidins, flavones, isoflavones, pterocarpan flavonoids, and flavanones, along with their respective glycoconjugate derivatives, were identified in leaf and root tissues. The synthesis of free isoflavones, followed by monoglycosylation and further malonylation was shown to be characteristic of root samples, whereas higher glycosylation, followed by further acylation with coumaric and ferulic acid was characteristic of leaf tissues. While minor changes were observed in leaves, significant quantitative changes could be observed in roots upon Hg treatment. Some flavonoids were strongly upregulated in roots, including malonylglucosides of biochanin A, formononetin and medicarpin, and aglycones biochanin, daidzein, and irisolidone. Hg tolerance appeared to be mainly associated to the accumulation of formononetin MalGlc, tricin GlcAGlcA, and afrormosin Glc II in leaves, whereas aglycone accumulation was associated with tolerance to Hg stress in roots. The results evidence the alteration of the flavonoid metabolic profile and their glycosylation processes in response to Hg stress. However, notable differences existed between varieties, both in the basal metabolic profile and in the response to treatment with Hg. Overall, we observed an increase in flavonoid production in response to Hg stress, and Hg tolerance appeared to be associated to a characteristic glycosylation pattern in roots, associated with the accumulation of aglycones and monoglycosylated flavonoids. The findings are discussed in the context of the flavonoid biosynthetic pathway to provide a better understanding of the role of these secondary metabolites in the response and tolerance to Hg stress in M. truncatula.
Keywords: LC-QTOF; Medicago truncatula; flavonoids; heavy metal stress; mercury; metabolomics.
Copyright © 2022 Alvarez-Rivera, Sanz, Cifuentes, Ibánez, Paape, Lucas and Pueyo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Alloway B. J., Alloway B. J. (2013). “Sources of heavy metals and metalloids in soils,” in Heavy Metals in Soils. Environmental Pollution, Vol. 22 ed. Alloway B. (Dordrecht: Springer; ), 11–50. 10.1007/978-94-007-4470-7_2 - DOI
-
- Arregui G., Hipólito P., Pallol B., Lara-Dampier V., García-Rodríguez D., Varela H. P., et al. (2021). Mercury-tolerant ensifer medicae strains display high mercuric reductase activity and a protective effect on nitrogen fixation in medicago truncatula nodules under mercury stress. Front. Plant Sci. 11:2268. 10.3389/FPLS.2020.560768/BIBTEX - DOI - PMC - PubMed
-
- Badiaa O., Yssaad H. A. R., Topcuogul B. (2020). Effect of heavy metals (copper and zinc on proline, polyphenols and flavonoids content of tomato (Lycoperdicon esculentum Mill.). Plant Arch. 20 2125–2137.
LinkOut - more resources
Full Text Sources

