Polygonatum Polysaccharide Regulates Macrophage Polarization and Improves LPS-Induced Acute Lung Injury through TLR4-MAPK/NF- κ B Pathway
- PMID: 35874106
- PMCID: PMC9303503
- DOI: 10.1155/2022/2686992
Polygonatum Polysaccharide Regulates Macrophage Polarization and Improves LPS-Induced Acute Lung Injury through TLR4-MAPK/NF- κ B Pathway
Abstract
Objective: To investigate the effects of polygonatum sibiricum polysaccharides (PSPs) on the polarization of macrophages to M1 and M2 phenotypes and their potential mechanism.
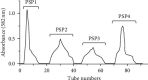
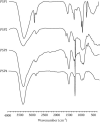
Methods: PSPs samples were prepared through water extraction and alcohol precipitation assay. The properties of PSPs were identified and analyzed by high-performance liquid chromatography, FT-IR, and NMR assay. Then, the effects of PSPs on mouse macrophage RAW264.7 viability were measured by CCK-8 assay. The cells were randomly divided into the control group, PSPs group, LPS group, and LPS + PSPs group. M1 phenotype polarization of RAW264.7 cells was induced by LPS treatment. The effects of various treatments on expression of M2 phenotype CD206, activation of TLR4-MAPK/NF-κB signal pathway, and translocation of NF-κB into the nucleus were determined by ELISA, western blot, and immunofluorescence assay, respectively. TLR4 inhibitor, TAK-242, and MAPK inhibitor, BIRB 796, were used to verify the effects of PSPs on the TLR4-MAPK/NF-κB pathway. The mice model of acute lung injury (ALI) was established and randomly divided into control group, PSPs group, LPS group, and LPS + PSPs group. Bronchoalveolar lavage fluid (BALF) and lung tissue were collected to measure protein, inflammatory cells, neutrophil and macrophage cells number, and the levels of IL-6 and TNF-α in BALF. Flow cytometry and western blot assay measured the phenotypic changes of macrophages and the activation of the TLR4-MAPK/NF-κB signaling pathway.
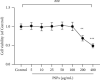
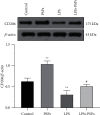
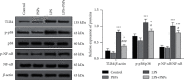
Results: The concentrations of PSPs lower than 100 μg/mL showed no toxicity to RAW264.7 cells. PSPs treatment could significantly reverse the reduction of CD206 protein expression (P < 0.05) and the increase of the expression of inflammatory factor TNF-α, IL-1β, and IL-6 (all P < 0.05), TLR4-MAPK/NF-κB signaling pathway activation (all P < 0.05), and NF-κB translocation into the nucleus induced by LPS. The effect of inhibitors TAK-242 and BIRB 796 was consistent with that of PSPs. In the mice model of ALI, PSPs treatment could reduce the total protein levels of BALF and the number of inflammatory cells level, reverse the number changes of neutrophils and macrophages, and downregulate the proinflammatory factors IL-6 and TNF-α caused by LPS (all P < 0.05). In addition, PSPs treatment could also significantly reverse the increase in the number of iNOS expressing macrophages in alveolar lavage fluid induced by LPS (P < 0.05). In contrast, CD206-expressed cells decreased (P < 0.05). PSPs could also reverse LPS-induced TLR4-MAPK/NF-κB signal pathway protein activation (all P < 0.05).
Conclusion: PSPs could suppress TLR4-MAPK/NF-κB activation induced by LPS, inhibit M1 phenotypic polarization of macrophages, and promote M2 phenotypic polarization, thus playing an anti-inflammatory role.
Copyright © 2022 Weizheng Zhou et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures






Similar articles
-
Syringic acid attenuates acute lung injury by modulating macrophage polarization in LPS-induced mice.Phytomedicine. 2024 Jul;129:155591. doi: 10.1016/j.phymed.2024.155591. Epub 2024 Apr 15. Phytomedicine. 2024. PMID: 38692075
-
Polygonatum sibiricum polysaccharides prevent LPS-induced acute lung injury by inhibiting inflammation via the TLR4/Myd88/NF-κB pathway.Exp Ther Med. 2020 Oct;20(4):3733-3739. doi: 10.3892/etm.2020.9097. Epub 2020 Aug 5. Exp Ther Med. 2020. PMID: 32855724 Free PMC article.
-
Essential oil from Cinnamomum cassia Presl bark regulates macrophage polarization and ameliorates lipopolysaccharide-induced acute lung injury through TLR4/MyD88/NF-κB pathway.Phytomedicine. 2024 Jul;129:155651. doi: 10.1016/j.phymed.2024.155651. Epub 2024 Apr 17. Phytomedicine. 2024. PMID: 38688144
-
Targeting different phenotypes of macrophages: A potential strategy for natural products to treat inflammatory bone and joint diseases.Phytomedicine. 2023 Sep;118:154952. doi: 10.1016/j.phymed.2023.154952. Epub 2023 Jul 12. Phytomedicine. 2023. PMID: 37506402 Review.
-
NF-κB: Governing Macrophages in Cancer.Genes (Basel). 2024 Jan 31;15(2):197. doi: 10.3390/genes15020197. Genes (Basel). 2024. PMID: 38397187 Free PMC article. Review.
Cited by
-
Revealing the active ingredients and mechanism of P. sibiricumm in non-small-cell lung cancer based on UPLC-Q-TOF-MS/MS, network pharmacology, and molecular docking.Heliyon. 2024 Apr 3;10(7):e29166. doi: 10.1016/j.heliyon.2024.e29166. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38617965 Free PMC article.
-
Ultrashort wave diathermy inhibits pulmonary inflammation in mice with acute lung injury in a HSP70 independent way: a pilot study.Mol Biol Rep. 2024 Jun 14;51(1):750. doi: 10.1007/s11033-024-09686-0. Mol Biol Rep. 2024. PMID: 38874700
-
Nanomaterials modulate tumor-associated macrophages for the treatment of digestive system tumors.Bioact Mater. 2024 Mar 20;36:376-412. doi: 10.1016/j.bioactmat.2024.03.003. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38544737 Free PMC article. Review.
-
Natural Compounds Regulate Macrophage Polarization and Alleviate Inflammation Against ALI/ARDS.Biomolecules. 2025 Jan 29;15(2):192. doi: 10.3390/biom15020192. Biomolecules. 2025. PMID: 40001495 Free PMC article. Review.
-
Structural Characterization of Polygonatum Cyrtonema Polysaccharide and Its Immunomodulatory Effects on Macrophages.Molecules. 2024 Apr 30;29(9):2076. doi: 10.3390/molecules29092076. Molecules. 2024. PMID: 38731567 Free PMC article.
References
-
- Yeung Y. T., Aziz F., Guerrero-Castilla A., Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Current Pharmceutical Design . 2018;24(14):1449–1484. - PubMed
-
- Zhang L. Y., Hei F. L. Emerging role of extracellular vesicles in lung injury and inflammation. Biomedicine & Pharmacotherapy . 2019;113108748 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

