Systemic Immune Modulation Alters Local Bone Regeneration in a Delayed Treatment Composite Model of Non-Union Extremity Trauma
- PMID: 35874126
- PMCID: PMC9300902
- DOI: 10.3389/fsurg.2022.934773
Systemic Immune Modulation Alters Local Bone Regeneration in a Delayed Treatment Composite Model of Non-Union Extremity Trauma
Abstract
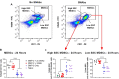
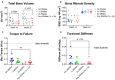
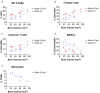
Bone non-unions resulting from severe traumatic injuries pose significant clinical challenges, and the biological factors that drive progression towards and healing from these injuries are still not well understood. Recently, a dysregulated systemic immune response following musculoskeletal trauma has been identified as a contributing factor for poor outcomes and complications such as infections. In particular, myeloid-derived suppressor cells (MDSCs), immunosuppressive myeloid-lineage cells that expand in response to traumatic injury, have been highlighted as a potential therapeutic target to restore systemic immune homeostasis and ultimately improve functional bone regeneration. Previously, we have developed a novel immunomodulatory therapeutic strategy to deplete MDSCs using Janus gold nanoparticles that mimic the structure and function of antibodies. Here, in a preclinical delayed treatment composite injury model of bone and muscle trauma, we investigate the effects of these nanoparticles on circulating MDSCs, systemic immune profiles, and functional bone regeneration. Unexpectedly, treatment with the nanoparticles resulted in depletion of the high side scatter subset of MDSCs and an increase in the low side scatter subset of MDSCs, resulting in an overall increase in total MDSCs. This overall increase correlated with a decrease in bone volume (P = 0.057) at 6 weeks post-treatment and a significant decrease in mechanical strength at 12 weeks post-treatment compared to untreated rats. Furthermore, MDSCs correlated negatively with endpoint bone healing at multiple timepoints. Single cell RNA sequencing of circulating immune cells revealed differing gene expression of the SNAb target molecule S100A8/A9 in MDSC sub-populations, highlighting a potential need for more targeted approaches to MDSC immunomodulatory treatment following trauma. These results provide further insights on the role of systemic immune dysregulation for severe trauma outcomes in the case of non-unions and composite injuries and suggest the need for additional studies on targeted immunomodulatory interventions to enhance healing.
Keywords: MDSCs; S100A8/A9; immune dysregulation; musculoskeletal trauma; non-union.
Copyright © 2022 Vantucci, Guyer, Leguineche, Chatterjee, Lin, Nash, Hastings, Fulton, Smith, Maniar, Frey Rubio, Peterson, Harrer, Willett, Roy and Guldberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

